Despite its ubiquity in human life, chemists have still barely unlocked what’s happening amid the flames. Kit Chapman reports
On 14 June 2017, the UK woke to images of a black vortex of smoke above a raging inferno. In west London, the 24-storey Grenfell Tower was on fire, the external cladding causing a stack effect – similar to a chimney – spreading its destruction throughout the building. It was the UK’s worst residential fire since the second world war, causing 72 deaths as residents became trapped on the upper floors. Fire scientist Claire Benson remembers watching the news that morning in horror. ‘I woke up that morning and I was furious,’ she recalls. ‘Because it shouldn’t have happened.’

Grenfell, along with other major blazes such as the destruction of Notre Dame Cathedral in France and the 2019/20 wildfires in the US and Australia, has shown how much work still needs to be done to understand how to prevent and control fires. Despite being one of the most familiar phenomena in most people’s lives, it’s surprising how little we truly know about how and why things burn.
Well worth the candle
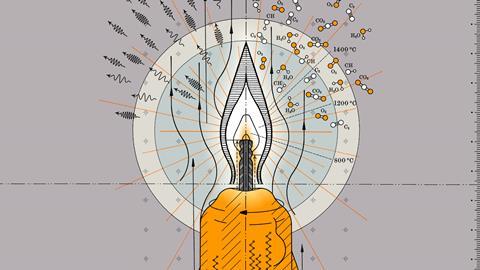
Although it’s more than 170 years since Michael Faraday’s lectures on the chemical history of a candle, we still struggle with a complete understanding of fire. A candle remains, however, a perfect place to start. This is a diffusion flame, where burning happens only at the interface between fuel and air – a region called the reaction zone, which is about 200µg deep. The interior of the flame itself is a completely oxygen-free environment.

But even this can be overly simple, explains Ludovico Cademartiri, an associate professor at the University of Parma, Italy. ‘If you spoke to someone in the street and said “What do you need for fire?” They’d say you need air, you need a fuel, you need combustion. That’s it. It’s not wrong, but it gives a false impression of simplicity. Combustion is the prototypical complex system,’ Cademartiri says. ‘The simplest hydrocarbon combustion – methane and oxygen – produces hundreds of different intermediates and byproducts through hundreds of different chemical reactions occurring at different rates. Those rates depend on temperature, which changes dramatically across a flame. Among the byproducts is soot, which is a solid with a sizeable heat capacity, so will get heated up and glow. For most people it’s hard to grasp how much complexity can hide beneath the reaction of just two reagents.’
This complexity is why fire remains so hard to grasp. While completing his postdoctoral work at Harvard University in the US, Cademartiri worked with fellow postdocs in George Whitesides’ lab and the US Defence Advanced Research Projects Agency to develop flame suppressants. At one point he asked one of the US Navy’s combustion experts when, on a chemical level, a fire is extinguished. ‘I asked if we can really determine the causal chain of events that lead to a specific flame going out. He said no.’
The simplest hydrocarbon combustion produces hundreds of different intermediates and byproducts
Between 2008–2011 the group’s task was to find alternative means to control fires in enclosed environments, such as the interior of an armoured car, where water damage can be just as devastating. Whitesides’ team focused on tactics to extinguish fires that wouldn’t require suppressants – and succeeded. ‘The combustion of hydrocarbons produces ions and electrons, which become part of the composition of the flame itself. So, effectively, part of a flame is a dilute plasma,’ Cademartiri says. By creating an oscillating, highly concentrated electric field, the team were able to deflect and extinguish flames about a metre tall. The idea proved so popular it was even used by DC Comics, with new Superwoman Lana Lang manipulating electricity to snuff out a fire and defeat Lex Luthor. Unfortunately, in the real world the gradient of the electrical field required meant it couldn’t be scaled up beyond flames with a base of a few centimetres.
The team were also able to use sound waves to suffocate the flame’s reaction zone by hindering convention of air to the flame, Cademartiri says. ‘But we needed very low frequencies, between 55Hz and 60Hz, at about 130 decibels. On the other hand, it could scale much better than the electric field. We thought about using it for something like a wildfire, just trying to contain it. But we were never able to do the feasibility study.’
Where the wildfires are
Wildfires are also diffusion flames and present an immense challenge worldwide. In 2019, the US National Interagency Fire Center reported 50,477 wildfires in the US, destroying more than 18,600km2 of land – a five-year low – with annual suppression costs totalling around $3 billion (£2.4 billion). It’s a level of devastation that atmospheric chemist Krystal Vasquez has witnessed first-hand. A PhD candidate at the California Institute of Technology, US, in 2019 she found herself among a team of other scientists and their instruments in the hull of a converted Douglas DC-8. They were flying out of Boise, Idaho to sample the smoke plumes of conflagrations raging across the Pacific Northwest. During an undergraduate internship, Vasquez took part in atmospheric sampling missions across central California and fell in love with flying fieldwork. When she found out there was a seat on a plane assessing wildfires for her PhD, she leapt at the chance to come aboard.

‘There was one fire that we went to that was toward the evening and you could see the glow of the flames and all of the smoke.’ Vasquez recalls. ‘It was pretty wild. The plane is doing zigzags across the plume, and you have to time everything so you can get that one or two seconds when you’re actually in the plume itself. Then you have the excitement of the cabin filling up with smoke, which is frightening. And you have the convection for the heat of the fire, so the plane is turbulent and bouncing. It’s like a fun rollercoaster… you learn to take a lot of [motion-sickness medication] Dramamine.’
The flight was part of the Firex-AQ mission, a joint venture between Nasa, the National Oceanographic and Atmospheric Administration (Noaa) and more than 40 partners to assess the complex chemistry of smoke. Vasquez’s role, with another colleague, was operating a time-of-flight ionisation mass spectrometer, which uses a fluorinated reagent ion that clusters with her target compounds. ‘On the flight the team measured aerosols, nitrogen oxides (NOx), hydrocarbons,’ she says. ‘I measured oxygenated hydrocarbons – the oxidation products that end up produced in smoke. Not to brag or anything, but they’re really difficult to measure because they are so reactive. We had to position our instrument at the window of the plane [which has a custom-built air inlet]; a lot of our compounds are super-sticky, such as nitric acid, so we needed to the inlet to be extremely short or they’d get stuck on the walls of the instrument.’
Much of wildfire smoke remains a mystery. Although a previous Firex mission identified the chemical mechanisms of furan-type compounds in the smoke, a large portion of the reactions are still unknown. Isocyanic acid (HNCO) was only found to be up to 30% of NOx in 2010, while there remains a large quantity of unidentified semi-volatile organic compounds in fires that impact modelling. And, even when they have been identified, how they work is not understood. For example, the NOx and volatile organic compounds emitted by the plumes undergo photooxidation but only sometimes produce ozone. This seems to depend on the precursors in the fire, the speed at which the plume cools and how efficiently the NOx is converted into products such as peroxyacetyl nitrate.
‘Wildfires can produce ozone and all these toxic gases,’ Vasquez explains, ‘but the compounds they produce is very dependent on the characteristics of the fire and the relative humidity. The main motivation [of Firex] is to understand the chemistry and composition of smoke plumes, so we can create models that forecast how fires impact air quality and maybe give first responders an idea of how fire might move. We also look at public health and land management, because a lot of farms end up using fire to just clear off the fields before next season. Being able to know what weather conditions are the most appropriate to do that in is important for air quality in the region.’
Dust devils
While blazes in the wilderness can produce a host of unpleasant gases, an entirely different airborne cocktail can be created in city fires. In Paris, France, restoration scientists from the Historical Monuments Research Laboratory are working in the gutted ruins of Notre Dame de Paris, the historic cathedral where a fire on 15 April 2019 shocked the world. The French government has since pledged to reopen the cathedral within five years, and researchers have pored over its exposed remains to understand historical processes. But from a health perspective, an even greater tragedy has unfolded than the lost art – as the strange yellow tinge to the fire’s smoke revealed.

During the inferno, the hundreds of tonnes of lead that lined the cathedral’s roof melted. It was on a scale never seen before – so much so that Parisian police warned people to wipe their homes and premises to avoid lead poisoning. It wasn’t until September that an investigation by the New York Times revealed the dust deposited around the cathedral was up to 1300 times higher than safety guidelines, with its spread covering much of central Paris.
Such toxic exposure happens every day around the world, albeit on a much smaller scale. Jennifer Keir is an environmental chemist and toxicologist at the University of Ottawa, Canada, who studies exposure of firefighters to heavy metals. The majority of studies have looked at firefighter exposure during training scenarios, Keir explains – but a burning wood pallet in a training house doesn’t reflect the variety of contaminants in a real fire. ‘We wanted to capture what they were being exposed to while on the job during emergencies,’ Keir says. ‘So we collaborated with the Ottawa fire services and looked at urine samples, wipes from their skin and air samples. We found they were significantly exposed. In terms of urinary metabolites, we saw huge spikes after firefighters attended a fire.’ Keir’s samples detected concentrations of metals such as antimony and lead on skin under the firefighters’ protective equipment, as well as increased levels of polycyclic aromatic hydrocarbons. ‘Some of these bioaccumulate,’ she says. ‘You’ll excrete a lot of them within days, but even when you’re excreting them, they’re still doing damage.’ Not all of the dangers are from the fire, Keir says. Often, protective equipment and firefighting foams include perfluoroalkyl and polyfluoroalkyl substances (PFAS), a family of fluorinated compounds used as flame retardants and surfactants but known to be detrimental to health. ‘Somehow that seems to be getting into firefighters as well,’ she notes.
Firefighters are significantly exposed to contaminants
Understanding exposure is only half of Keir’s work. As firefighters can’t prevent exposure – air flow is needed to keep cool and prevent them overheating during a call-out – she focuses on post-fire decontamination. ‘There’s this movement in the fire service that you immediately wipe down after a fire, because we know about skin exposure. There have been companies promoting wipes to clean skin before firefighters get back to the truck, before they can get back to the station and shower. But there’s been no real scientific studies to look at this, and whether those decontamination methods actually remove a significant amount of the hazards they are exposed to.’ Keir’s work is currently on hold due to the Covid-19 pandemic, but once complete will help protect firefighters when fires do break out.
Razing the standard
Perhaps the greatest role for fire scientists is preventing blazes to begin with. Chemists have been involved in fire safety throughout history – from Faraday’s mentor Humphry Davy’s role in safety lamps through to the creation of americium-241, an isotope that does not exist naturally on Earth, for use in smoke detectors. It’s this drive for safety that left Benson so aghast as she saw the Grenfell disaster unfold. A senior lecturer at London South Bank University, Benson’s primary interest is fire in low pressure environments, but she has also worked with the London Fire Brigade and has an interest in the chemistry of materials used for buildings. The problem is that safety standards are just as complex as fire itself.
‘I have a lot of sympathy for people who have to put standards together,’ she explains. ‘For sofa materials, we can say it has to resist a flame of a certain wattage for a certain period of time. We do the same for building materials. The problem is that there are lots of different characteristics you could choose. It’s one of those things that, while you could say something doesn’t burn rapidly, it could still give off smoke. And even just burning itself is a problem – are you talking about resisting ignition, that it shouldn’t ignite until a certain point? Different materials may have a really high auto-ignition temperature, but ignite really quickly when exposed to a naked flame.’
This is just the first hurdle, Benson says. Materials might form carbon char layer, caused by incomplete combustion, so that even if they do catch fire they go out quickly. This could be safer than a material that takes longer to ignite, but then won’t stop burning. ‘Then you’ve got flame speed across the surface of the material. And heat release rate, when a material releases a lot of energy quickly, which could be more dangerous in terms of onward combustion.’
I have a lot of sympathy for people who have to put standards together
The UK Fire Protection Association’s (FPA) critique of the tests carried out on Grenfell Tower’s cladding raised several concerns. The insulation was made from polyisocyanurate, and was tested by creating a three storey tower. ‘At the bottom, they created a wooden crib, a lattice of wood,’ Benson says. ‘It was used so there was a known heat release rate, so we can extrapolate back and it’s a nice, clean comparable test. But the point the FPA made was that it wasn’t representative of the environment because so much of it is plastic, which has a much higher heat release rate [than wood] … another excellent point is that the test was done inside a very large warehouse, where there’s no air flow. And I don’t know if you’ve ever put your hand outside a tower block window, but the wind 20 or 30 storeys up is very different.’
The hope is that these standards can be updated to better reflect the real world – and prevent another disaster such as Grenfell. It’s a mission that will require chemists and materials scientists working in partnership with fire services and architects. And even then, we’ll have barely begun to understand the mysteries of fire – and tackle the immensely complex scientific challenges it poses.
Kit Chapman is a science writer based in Southampton, UK












No comments yet