Electric vehicles are surging in popularity but Patrick Hughes asks what happens once their batteries are no longer fit for purpose
With the electric vehicle (EV) industry poised to grow exponentially, so too is the waste from the lithium-ion batteries that power these vehicles. From the EVs sold globally in 2017 alone, the waste from the spent lithium-ion batteries could be about 250,000 tonnes, or a half a million cubic metres – that’s enough to fill the Royal Albert Hall in London almost six times. With the average battery only guaranteed to last eight years, some of those 2017 batteries could need to be replaced and disposed of by 2025. As more batteries are manufactured to keep up with the increasing demand for EVs, the recovery of the critical materials in these batteries will be vital to create a circular, sustainable industry and to manage waste. But currently, no recycling method exists which is both efficient and profitable enough to be sustainable. What is to be done with the next generation of end of life batteries?
Why recycle?
One of the greatest issues driving lithium-ion battery recycling is one of waste management – but it is not correct to say that all of the batteries being manufactured today are headed for landfill. When a lithium-ion battery comes to the end of its life, it still retains around 80% of its charge – and while that’s not enough to serve an electric vehicle, it’s good enough for a variety of different applications, such as energy storage. These second-life batteries could be used for at least 10 years. This kind of reuse is preferable in the first instance to recycling, because they’re extremely valuable – and recycling is costly. ‘The material in a Tesla battery, for example, is worth around $1500 (£1200) – but the market value is between $10,000 and $15,000,’ says Hans Eric Melin, founder of Circular Energy Storage, a consultancy that researches the life-cycle of lithium-ion batteries. ‘If you want to get hold of that material value, you have to disassemble it, you have to crush it, you have process it in some kind of recycling process. And then you only have a couple hundred dollars.’
The demand for these battery packs is large – the world’s biggest operator of telecommunication towers, China Tower, intends to replace the lead-acid batteries used for back-up power at almost all of their 2 million tower base stations with second-life lithium-ion batteries. That’s 54GWh of battery storage – or around 2 million batteries. In this way, reuse can play a role in adding value and providing the recycling industry with time to pull together the necessary infrastructure to recycle batteries at scale. But with the total amount of lithium-ion batteries expected to reach 7.8 million tonnes per year by 2040, according to a report from IDTechEx, it’s anticipated that global supply of end of life batteries will exceed their demand in second-life applications. And not all batteries will be capable of reuse – those of unknown provenance, or those involved in a crash, will need recycling right away. And of course, batteries do eventually die for good – so a far-sighted approach for recycling is important in the long run in managing waste and bolstering reserves of critical materials. ‘The eventual fate for all LiBs at the end of their first and potentially second lives should be recycling,’ says Gavin Harper of the University of Birmingham’s Centre for Strategic Elements & Critical Materials in the UK.
Since these batteries often contain valuable materials such as nickel, manganese and cobalt, to eventually dump them in landfill as volumes of waste increase would be a waste of precious resources. ‘If the purpose of having electric vehicles is to reduce carbon dioxide emissions, then extracting these raw materials from the ground to produce the EVs goes against the purpose of having them,’ says Zubera Iqbal, a researcher working on battery recycling at the University of Birmingham. A solid recycling infrastructure would also slow depletion of the critical cobalt reserves necessary to manufacture these batteries – it’s estimated that by 2030, recycling could provide Europe with 10% of its cobalt supply. That provides the industry with an added benefit of relying less on problematic sources: at least 60% of the world’s cobalt is mined in the Democratic Republic of the Congo, where some of the most vulnerable in society bear the brunt of its extraction – cobalt mining in DR Congo is linked to human rights abuses, such as child labour and armed conflict.
Difficulties in recycling
Much of the recycling that does take place today is done through a combination of pyrometallurgy and hydrometallurgy – a process that leaves more to be desired. While the process recovers some of the most valuable metals in the battery, much of the other valuable material is lost. ‘Basically, you throw the battery into a smelter, and you get a mixture of alloys out the bottom – typically nickel, cobalt and copper,’ says Linda Gaines, an expert in materials and life-cycle analysis at Argonne National Laboratory in the US. ‘The lithium and aluminium get oxidised and go to the slag – they aren’t economical to recover.’ After the lithium and aluminium gets sent to landfill, you’re left with the metal alloys – these then have to be treated hydrometallurgically to extract the maximum value, breaking down the cathode’s crystal structure and leaching the different ions out of the battery to end up with the precursor salts like nickel sulfate and cobalt sulfate which you can use to manufacture new batteries. ‘Cobalt is by far the most valuable product,’ says Gaines. ‘But as automakers are moving towards battery chemistries with less and less cobalt in them, the valuable product you’re getting out of the leaching process keeps decreasing.’

Numerous issues complicate the development of a more efficient process. A fundamental problem is that these batteries simply aren’t designed to be recycled, they’re designed for high-performance and longevity – essential capabilities of a battery which could be impaired at the expense of designing a battery that is more recyclable. A lithium-ion battery pack is made up of several thousand of cells grouped in modules, with each cell containing a cathode, anode, separator and electrolyte. Cathodes generally consist of an active transition metal oxide powder mixed with carbon black and glued to an aluminium-foil current collector with a compound such as poly(vinylidene fluoride) (PVDF). The anodes contain graphite glued to a copper foil with PVDF, and the electrolyte is usually a solution of LiPF6 salts.
It’s this large number of components, as well as the way that they’re put together, makes separating them difficult. ‘Battery packs aren’t designed for taking apart,’ explains Emma Kendrick, chair of energy materials at the University of Birmingham. ‘You end up with things like very strong glues used in assembly, which you then have to find some way of removing to try and take the cells out of the module. Or, you have several cells that are welded together, and you have to try to remove the welds from them. In that way, they’re not really designed for disassembly,’
Because of that, the cheapest and most common way to recycle batteries is to shred them. But no standard electric vehicle battery exists – popular chemistries include nickel cobalt aluminium, lithium manganese oxide, and nickel magnesium cobalt, among others, and every car model has a different battery pack structure. ‘If you’re recycling, you can’t control what you get, so all these batteries are going to come in together,’ says Jack Vaughey, a chemist at Argonne National Laboratory. When shredding batteries, these different compounds have to be separated. That can be done with density separation, or by exploiting the differing magnetic properties of the different metal oxides.
The way that packs are assembled also means that after shredding, one of the most valuable components – the cathodic ‘black mass’, or active electrochemical metal oxide – comes out contaminated with other materials. To combat that, researchers are looking at how they can design packs, modules and cells in such a way that they are easier to take apart at the end of their lives to circumvent the need for shredding, or to make purification simpler. That can be as simple as replacing welds with nuts and bolts, or attempting to develop new glues which are easier to work with. ‘If we’ve got a system in 10 years’ time that’s still using exactly the same batteries we’ve got at the moment, then it’s going to be a disaster,’ says Andrew Abbott, professor of physical chemistry at the University of Leicester in the UK. But batteries are designed by manufacturers – and being recycling friendly doesn’t always go hand in hand with their performance standards. ‘We find it very challenging to get people to say “We can do this” – there’s a lot of pushback,’ says Jeffrey Spangenberger, the director of the ReCell recyling programme at the Argonne National Laboratory.
The state of recycling today
Even with a lack of design for recycling, researchers are looking at ways to maximise recovery. A purely hydrometallurgical process would be more efficient– that is, using aqueous solutions to leach out the valuable metals from cathode material after shredding. That’s usually carried out using a combination of sulfuric acid and hydrogen peroxide, with the peroxide working as a reducing agent to convert insoluble Co(iii) materials into soluble Co(ii). After leaching, the cobalt and lithium can be recovered as salts through precipitation by changing the pH of the solution. That process leaves you with high purity starting materials which can then be used to manufacture new batteries. But while hydrometallurgical processes recover more of the valuable materials than purely pyrometallurgical ones, the value that comes from the actual cathode material is lost. That means that while it’s useful for cathodes which contain a large amount of cobalt like lithium cobalt oxide – hydrometallurgical processes can recover up to 70% of these cathode’s value – it’s less useful for cathodes which are less cobalt-rich, where most of the value lies in the manufactured cathode oxides themselves, rather than in the raw materials.

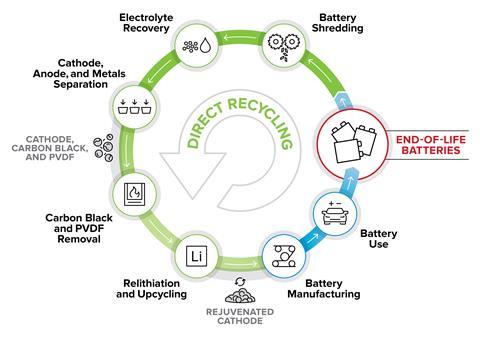
In cases where the value of the raw materials is lower, it is possible to recover the valuable cathode itself. Through a process known as direct recycling, this is what ReCell – a programme based at the Argonne National Laboratory and consisting of around 50 researchers at six national labs and universities – is exploring. ‘Direct recycling is based around preventing the crystal structure of lithium-ion battery cathode from being broken down – the benefit being that you maintain the value of what goes into transforming the raw materials into a cathode, which can be quite significant,’ says Spangenberger, the programme’s director. Direct recycling has another benefit, in that it also allows other valuable components such as aluminium and copper foils, salts from the electrolyte and graphite from the anode to be recovered. In direct recycling, the battery is again shredded and the black mass – a mix of cathode and anode powders – recovered. ‘When you get the powders back, they’re coated in polymers – the glue that attaches them to the metal foils,’ says Vaughey, who is coordinating the direct recycling effort within ReCell. Those powders have to be carefully purified without damaging the active powder surface – for example, removing the glue using a solvent or by depolymerising with heat.
While direct recycling recovers the highest value product in principle, recyclers need to consider why the battery needs recycling in the first place. Oftentimes, the active cathode material has degraded – and for it to be useful again, it needs to be reinvigorated with new lithium. Using spectroscopy, you can make a rough estimation of how much lithium is missing, and new lithium can be added by mixing the cathode with lithium hydroxide and heating to 220°C. And a rapidly changing market means that manufacturers are always shifting to newer chemistries with better capabilities. To repurpose cathodes recovered in this way, it can be necessary to upgrade them. Older nickel magnesium cobalt batteries, for example, contained a much lower proportion of nickel than new iterations. To recover something which the market will actually want to buy, it’s necessary to increase the nickel content of the recovered material . That’s a new area that Vaughey and his team are exploring – one difficulty is in making the nickel homogeneous throughout the cathode. ‘The speed at which something diffuses is related to its charge – the more highly charged something is, the more slowly it diffuses,’ says Vaughey. ‘In this case, we’re trying to move 3+ cations into the solid back and forth and get them to be homogeneous – which is pretty tough.’
But not everyone is shredding batteries. In the UK, the Faraday Institution’s Recycling of Lithium Ion-Batteries (ReLiB) project is taking a leaf out of what some companies are trying to do in the far east – taking the pack and splitting it open into the anode, cathode and separator to end up with a much purer stream of material. ReLiB are developing an automated system for disassembly in this way which would remove the risk of electric shocks for technicians performing manual disassembly. ‘All we’ve got to do then is to separate out the metal foils from the active material surface,’ says Andrew Abbott, a co-investigator at ReLiB. To that end, the team at ReLiB has devised a delamination method using ultrasound, capable of separating the cathodes from the metal current collectors 100 times faster than using acids – in a matter of seconds, rather than hours.
ReCell and ReLiB are not alone in their efforts. More and more recycling research networks devising their own innovations and techniques continue to appear and grow all over the globe. As recycling infrastructure comes together to recycle at scale, it isn’t yet clear which one of these methods, if any, will be the preferred route to take. ‘You need to understand the value of your waste, and trade that off against how expensive your process. And I’m not sure we have that answer yet,’ says Kendrick. What may be the case is that these different methods will be used in conjunction, according to what is most profitable and what works best for different batteries. But in order to give the recycling industry the greatest chance of success, Abbott thinks it’s important that these projects share their expertise. ‘What’s going to happen over the next 10 years is that there’ll be a cross fertilization of ideas,’ says Abbott. ‘It’s really important that these projects don’t work on their own.’
Patrick Hughes is a science writer based in Belfast, UK













1 Reader's comment