Tackling climate is not just a job for scientists in more developed countries. Munyaradzi Makoni talks to researchers in Kenya and South Africa to find out more
With climate change already affecting many countries across Africa, chemists there are investing their expertise to make an impact on climate adaptation and environmental problems. From designing enzymes for green manufacturing, exploring batteries that can store more renewable energy to turning lake weeds into green fuel are some of the initiatives under investigation or already delivering the promise of new tomorrow.
Improving on nature
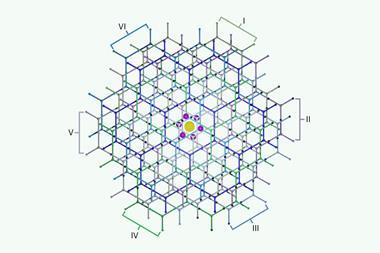
In 2019, Jeremy Woodward, Andani Mulelu and Angela Kirykowicz, biologists from the University of Cape Town, South Africa, delivered novel and exciting insights into the structure and inner workings of a plant nitrilase enzyme – a member of a class of enzymes that are widely used in the synthesis of a broad range of chemicals. Some of the enzymes in this family could one day clean up toxic cyanide waste.
The 3.4Å resolution structure of the protein was obtained at the electron bio-imaging centre at the UK’s national synchrotron – Diamond Light Source. The Synchrotron Techniques for African Research and Technology (Start) project, funded by a £3.7million grant from the UK’s Science and Technology Facilities Council, opened the passage to Diamond for African scientists working in the fields of structural biology, catalysis and energy materials. The grant supported post-docs, workshops, exchange visits and a structural biology resource centre at the University of Cape Town. During the grant period from 2018 to September 2021, over a hundred scientists from Africa and UK collaborated through exchange visits and training.

The nitrilases are a member of a superfamily of enzymes that include some amidases that have been studied at the University of Cape Town for over 30 years. While the amidases occur as dimers that often group together in symmetric assemblies containing more subunits, the nitrilases assemble into long helical filaments.
While working on plant nitrilases in the laboratory of Markus Piotrowski at the Ruhr-University Bochum in Germany 10 years ago, Woodward observed that the substrate specificity could be modified, not only by modifying amino acids near the active site, but also by altering amino acids in a ‘hot spot’ far from the active site. This latter alteration changed the helical pitch of the filament.
Even before working as a research fellow, Mulelu had been fascinated by the structure of helical nitrilases during his final year as an undergraduate, especially those that detoxified cyanide, which have potential to clean up pollution from mining and electroplating. During his doctoral studies he worked on a bacterial cyanide hydratase enzyme, a member of the nitrilase superfamily known to specifically speed up the conversion of cyanide into formic acid and ammonia. Mulelu determined the structure at near atomic resolution (3.15Å) by cryoEM together with Julian Reitz at the Goethe University Frankfurt in Germany. A significant difference between the plant and bacterial nitrilases was an insertion of only five amino acidsin an interfacial surface of the helical subunit near the ‘hot spot’ identified by Woodward.
Woodward and Kirykowicz decided to explore this systematically by targeting selected amino acids for directed evolution. This technique connects the survival of bacteria expressing nitrilase variants to the successful conversion of substrates and thus enables a detailed analysis of the correlation between substrate specificity and amino acid variation at specific locations.
Mulelu and Woodward asked the question: ‘How did this work? How did these translate into differences that could account for the ability of these enzymes to distinguish between substrates that differ by only one carbon atom?’
The answer lay in determining the structure by cryoEM. The GCRF Start grant enabled Mulelu and Woodward to travel to the electron bio-imaging centre at the UK’s Diamond Light Source with their precious samples and return with terabytes of image data. The result was a three-dimensional map of stunning clarity which was easily interpreted. ‘We saw the enzyme at high enough resolution to build in the coordinates of the individual atoms in the protein,’ Mulelu says.
The results show how the insertion creates a flap over the active site of an adjacent subunit in the helix enabling one of the ‘hot spot’ side chains to penetrate the heart of the active site of the neighbouring subunit. Thus the specificity pocket is dependent on the interaction of two adjacent subunits in the helical assembly and enabling the specificity to be tuned by modifying amino acids that are far removed in sequence space from the catalytic site.
These results set the tone for new research possibilities. Woodward had his sights on ‘designer’ nitrilases’ to find biotechnology solutions which could lead to sustainable changes in the lives and livelihoods of Africans. A major challenge in protein engineering is the generation of enzymes with novel substrate specificities. A detailed knowledge of several nitrilase fibres will enable in silico generation of nitrilase variants with tailored specificity and other properties.
Cleaning up cyanide
This approach has been adopted by Lenye Dlamini, currently doing her PhD in the structural biology research group at the University of Cape Town. She picked up Mulelu’s work and that of Michael Benedik’s group at Texas A&M University in the US who used directed evolution to increase protein stability. Her goal is to use computers to rapidly identify specific amino acid changes that will lead to increased stability of the cyanide degrading enzymes and thus will make them better agents for the control of environmental cyanide pollution.
As efforts to reduce human impact on the environment demands more sustainable and environmentally friendly waste management systems, as well as the bioremediation of damaged ecosystems, biocatalysis can be a replacement for conventional chemical synthesis in industries in line with the UN’s Sustainable Development Goals.
Dlamini says the engineering of a cyanide-degrading nitrilase enzyme can be used to reclaim cyanide waste in the textile, electroplating and gold-mining industries, which use cyanide and produce cyanide-containing waste. Spills or unsafe disposal results in environmental pollution causing damage to natural resources, ecosystems and poses a threat to human health.
The direct impact of Dlamini’s research project is environmental, but it has broader implications in a variety of industries. The demand for biological agents and processes that will replace conventional processes of managing waste and chemical synthesis is also growing. ‘Enzymes, including the nitrilases, have taken centre-stage in this regard and present not just an environmentally benign alternative but one that produces better reaction products,’ she says.
Nitrilases are highly specific in the enantiomers and regions on compounds that they bind to, leading to more specific reaction products. Using predictive biophysical methods based on structural knowledge of cyanide degrading nitrilases, Dlamini intends to measurably improve their tolerance of non-optimal temperature ranges and enhance the operational stability of the enzyme so that they can be routinely used for safe and cheap disposal of cyanide waste.
New materials for lithium-ion batteries
Although widely used around the world in personal devices, and increasingly in use in electric cars, rechargeable batteries face a range of problems. The most widely used types contain toxic electrolytes such as sulfuric acid in lead acid batteries and lithium perchlorate in lithium-ion batteries. Lithium-ion batteries are expensive, and their components can deteriorate over relatively short periods of time raising reliability concerns. The constant replacement of these materials has a negative impact on the environment.
Commercial batteries use a lithium salt, such as LiClO4 or LiFP6, dissolved in an organic solvent, commonly alkyl carbonates as an electrolyte and these organics compromise the safety of the battery. Solid-state electrolytes would eliminate the need for a separator, however, avoid the use of organic electrolytes and therefore enable safer batteries that do not pose any leakage risks.
With South Africa being of the biggest polluters, it is important that we work on renewable energies
Now, Gugulethu Nkala, a PhD student at the school of chemistry at the University of the Witwatersrand in Johannesburg, South Africa, is working on a material based on the sodium superionic conductor (Nasicon) structure type, namely lithium titanium phosphate.

She is investigating the effects of various dopants on the structure and properties of lithium titanium phosphate compounds as potential materials for solid-state electrolytes in lithium-ion batteries to address the challenges that arise from current batteries.
Nkala aims to understand how the partial replacement of Ti4+ in LiTi2 (PO4)3 with +3 and +4 metal cations affects its conductivity of lithium ions at different temperature and under different atmospheres, specifically air and nitrogen. The research also involves exploring ways to lower the cost of batteries.
Substituting Ti4+ with trivalent cations such as Al3+ increases the amount of charge carriers. The other strategy entails altering the channels through which Li+ cations migrate in the structure by substituting with larger cations, such as In3+ or Zr4+.
Nkala says the project has focused on investigating the structures – both local and average – of these variously substituted materials with techniques such as powder x-ray diffraction (PXRD), Raman spectroscopy, pair distribution function (PDF) and x-ray absorption spectroscopy (XAS).
‘Although the in-house techniques (PXRD and Raman spectroscopy) have provided insight into the phases present as well as an average structure understanding, the local structure of these materials is seldomly studied experimentally,’ she says.
Nkala says access to synchrotron facilities such as Brookhaven National Laboratory in the US and Diamond Light Source, through funding bodies such as the GCRF Start, has been invaluable for higher resolution studies. The PDF and XAS techniques have been instrumental in providing insights on the mechanisms that govern the changes in observed ionic conductivities. Access to characterisation techniques such as PDF and XAS have revealed that the local structure may deviate from the average structure obtained via PXRD.
Nkala and her team are currently working on reconciling the local (from PDF and XAS) and average (from PXRD) structure models, to provide a holistic picture of the influence of structure on the properties in these Nasicon-type materials.
Thinking local
In line with the UN’s Sustainable Development Goals, Nkala hopes to narrow the energy poverty gap in Africa. One of the ways scientists can narrow the gap is through fostering community understanding of the work they do in the Energy Materials Research Group. Prior to the Covid-19 pandemic, they were participating in community outreach events such as the Sasol Techno X and Yebo Gogga expos, showcasing their work in an easily digestible manner to primary and high school students.
Under the supervision of Dave Billing, Caren Billing and Roy Forbes, Nkala wants to improve renewable energy storage systems to make them more efficient, affordable, safe and environmentally friendly.
If the raw materials are locally available it will reduce the cost of battery production
‘With South Africa being of the biggest polluters due to the predominant power generation through combustion of coal, it is important that we work on solutions to drive the use of renewable energies,’ says Caren Billing. South Africa’s landscape makes it ideal to harvest solar and wind energy but these alternative energy sources are uncontrollable and intermittent, thereby driving the need for efficient and reliable energy storage devices.
Lithium-ion batteries play an important role in this as they have high energy density and relatively simple reaction mechanisms, she says – adding that lithium-ion batteries are widely used and that is only predicted increase. ‘This adds the need for producing safer batteries and the reason we are investigating solid state electrolytes,’ Nkala says.
Efficient energy storage
Michelle Sibonokuhle Nyoni was working in the farming sector in Zimbabwe when a thought occurred to her. ‘I realised that we are blessed with abundant renewable sources of energy – wind and solar – yet hindered by the challenge of how to store this energy effectively,’ she recalls. The idea of energy storage batteries was ignited and it became the focus of her PhD in 2017.

While working as a chemistry lecturer at the Chinhoyi University of Technology in Zimbabwe, she is now a part-time PhD student at the University of the Witwatersrand investigating lithium vanadium phosphates as improved cathode materials for lithium-ion batteries.
South Africa is one of the biggest vanadium producers in the world; Zimbabwe is one of the biggest lithium producers in the world. ‘If the raw materials are locally available it will hopefully mean reduced cost of battery production,’ she says.
Nyoni aims to understand what is happening at the atomic level. ‘What is happening with the structure of the material? Lithium vanadium phosphate materials have been made, but is this synthesis method reproducible? Can it be up scaled to commercial levels? How does adding a dopant manipulate the electrochemical properties of the material?’
The potential of lithium vanadium phosphate lies in its various attractive properties, including a high thermodynamic and kinetic stability. It can also have very good electrochemical properties, means they will have high specific energy, high working voltage and good cycle stability.
As batteries age and they undergo more cycles of charging and discharging, the capacity gets poorer but lithium vanadium phosphate materials have good cycle stability. A key application is in electric vehicles, which will benefit from increased length of travel due to the cycle stability of lithium vanadium phosphate materials.
‘The lithium vanadium phosphate materials that I am working with are cathode materials, therefore if we can source these locally within the Southern Africa Development Community region of Africa, this would make them a lot more affordable and accessible,’ she says.
So far, Nyoni has used powder x-ray diffraction and Raman spectroscopy to study her samples to gain a clearer understanding of the chemical structure, phases and interactions. Unfortunately, due to Covid travel restrictions, the experimental work for her PhD has been hindered but she is looking forward to getting back in the lab from December 2021.
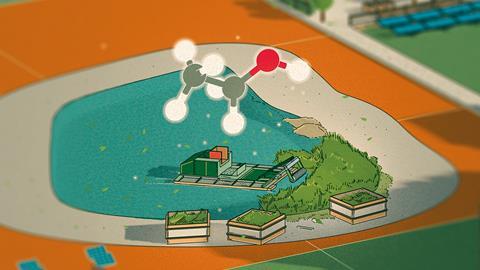
The weeds of change in Kenya
Kenya’s cooking fuel market is dominated by charcoal, firewood, liquefied petroleum gas and kerosene. These pose serious health hazards, environmental and socio-economic costs. Globally, 3 billion people cook over smoky open fires or on stoves using kerosene, wood, charcoal, dung or coal, the World Health Organization says. As a result, more than 4 million deaths occur every year. Dirty cooking fuels also contribute to climate change.
Our business solves two problems, the water hyacinth menace and energy poverty
But clean modern cooking fuels are gaining traction in Kenya. Among the transformative fuels considered viable, clean and affordable is ethanol, though still on a small scale. Just 1.2 million litres are produced annually in the country.
Ethanol can be produced from a variety of feedstocks. Sugary or starch-bearing plants such as corn, sugarcane, sweet sorghum, barley and cassava are the prime candidates.
In Kenya, the bulk of the ethanol comes from sugarcane, but ethanol producers must compete directly with the sugar manufacturing industry, as both use it as a raw material and sugarcane production is too small. In a case where there is poor quality of crops yields are small, resulting in shortages and price volatility. Cassava could have been another option, but Kenya’s cassava value chain is poorly developed, leading to poor yields. Furthermore, cassava is prone to diseases including brown streak virus. The plant’s roots rot within 24-48 hours if not stored properly and could mean significant losses if not stored efficiently before ethanol processing.

Water hyacinth, an aquatic floating plant native to South America, could be better alternative. It was first detected on Lake Victoria in the early 1980s, and by the mid-1990s it had established itself, covering about 17,000 hectares on the Kenyan side of the lake. Hyacinth’s abundance is giving it an edge among other raw materials for ethanol production.
Back in 2016, Richard Arwa was a chemistry teacher at a secondary school. He and two students started extracting fuel from hyacinth at an experimental level and produced a small sample for a science competition. The sample took them from a local competition to the national level. At each level comments from judges encouraged them to polish the idea into an opportunity for business.
Arwa now runs the Centre for Innovations Science and Technology in Africa in the lakeside city of Kisumu. ‘When we started our business our aim was not to solve the problem of water hyacinth, but as we moved on our business solved two problems, water hyacinth menace and energy poverty,’ says Arwa.

Just over half of a water hyacinth plant is cellulose, a key ingredient in the production of bioethanol. The process starts with hired boats being used to manually harvest the abundant weeds, known for obstructing fishing, a key livelihood around the lake. The roots and the leaves are cut off, and only the stem is transported by trucks to a factory where the weed goes through a series of procedures to separate compounds such as hemicellulose and lignin from the cellulose.
Water hyacinth has strong cellulose bonds. Arwa manufactured an enzyme which breaks these bonds through fermentation. Through hydrolysis, a process to extract fermentable sugars, the cellulose is made to react with water. The sugars are then fermented, converted into ethanol and distilled to a high concentration. Up to 94% pure ethanol is achieved through this process.
To separate and distil the ethanol Arwa built his own equipment after finding that commercially available equipment, which produces 2000 litres of ethanol per day, was unaffordable. He plans to manufacture and sell the equipment to other small businesses.
Clean and cheap
Compared to other commonly used fuels in Kenya, such as kerosene, CIST’s ethanol is nearly 40% cheaper than kerosene. Lab tests have shown that when CIST ethanol burns, it emits a negligible amount of smoke and has a low sulphur content making it safe for cooking.
To produce a litre of hyacinth biofuel roughly costs £0.27, which retails at £0.46, cheaper than the £0.55 or more for a litre of paraffin. A litre of water hyacinth biofuel burns for up to five hours on the highest power setting. A litre of biofuel lasts for around three days for a household preparing three meals a day, adding up to around 10 litres at £4.60 per month.
Amongst 196,000 registered refugees and asylum seekers in western Kenya at Kakuma refugee camp are happy customers. They buy the ethanol cooking fuel which they also use the fuel for lighting. Entrepreneurs in the refugee camp buy and resell it to make an income.
As of early this year CIST-Africa was producing 600 litres of ethanol fuel daily with plans hopes to double it by year-end. Arwa says they are currently constructing laboratories to assess the quality of their raw material and ethanol they produce. Plans are also in line to produce 12,000 litres a day, targeted to increase to 60,000 litres a day in 5 years.
Capturing carbon in Zimbabwe
In 2019, Gift Mehlana, a natural scientist at Midlands State University in Gweru, Zimbabwe, won a £300,000 grant from the Future Leaders – African Independent Research (Flair) programme. It ignited his desire to set up a first research group on carbon dioxide capture in the country.
Flair, organised by the Nairobi-based African Academy of Sciences and the UK’s Royal Society, gave him the space, time and resources to do his research. He recruited two masters and three PhD students. ‘I felt great to be given such an opportunity,’ says Mehlana, who is the current president of the Zimbabwe Chemical Society.
At the heart of his plan was to arrest the increase of carbon dioxide concentrations in the atmosphere. Mehlana’s team followed two approaches known for efficiency in converting carbon dioxide to methanol or formic acid, using chemical and biological catalysts. It meant using small molecules that contain ingredients that react with greenhouse gases to change them into useful chemicals to build everyday products. They developed a specialised surface in their lab which they use to capture carbon dioxide and turn it into formic acid, a potentially useful chemical and feedstock that might even find use as a hydrogen storage carrier.
We have prepared a number of novel materials that we have applied in capturing and converting carbon dioxide to formic acid
‘We chose adaptable materials that easily allow liquid and air to pass through them because they can be easily fine-tuned to achieve desirable properties,’ he said. The team managed to produce formic acid and methanol, which can be blended with gasoline to improve air quality and used to make other clean-burning fuels.
The funding from Flair also provided a powder x-ray diffractometer for analysing materials the first of its kind in Zimbabwe. This greatly improved Mehlana’s set-up: before this, the lab’s samples were sent outside Zimbabwe for analysis, and then sent back, severely hampering his ability to do research.
In 2020, Seeding Labs donated some water purification systems, chillers and some basic chemistry equipment to Midlands State University’s Chemical Department. Analysis is no longer an obstacle to developing technology for recycling carbon dioxide.
‘We have prepared a number of novel materials that we have applied in the capturing and conversion of carbon dioxide to formic acid,’ Mehlana says. ‘We need to implement this on a large scale to determine its suitability in reducing the amount of carbon dioxide that is emitted into the atmosphere from power plants and cement manufacturing companies,’ he adds.
With climate change and environmental damage disproportionately affecting people in lower-income countries, it is vital that scientific and technological solutions to the problems they face are developed. And who better to understand and fix those problems than people in the countries themselves?
Munyaradzi Makoni is a science writer in Cape Town, South Africa










No comments yet