There’s still a lot we don’t know about the biogeochemical cycling of sulfur, and this could impact our ability to correctly model the climate. Rachel Brazil talks to the researchers trying to fill in the gaps.
It’s the fifth most abundant element in the universe, the 15th most abundant in the earth’s crust and it’s essential for life, but sulfur ‘seems to be the [element] that people forget about’, says chemist Spencer Williams from the University of Melbourne in Australia. As with carbon, nitrogen and phosphorus, its use and chemical conversion through the physical and biological world is described in a cycle. But some of that cycle is not well understood, particularly how small organo-sulfur molecules are produced and used in the oceans.
One of the main reasons for the renewed interest in the sulfur cycle is its influence on climate, from ocean emissions of dimethyl sulfide (DMS). It was once thought that sulfur was released from marine algae and microbes as hydrogen sulfide, but we now know that approximately 300 million tons of DMS are released from the oceans annually. ‘The smell of the ocean that we’re all familiar with is very low levels of DMS,’ says Williams.
It was UK environmental scientist James Lovelock who proposed the idea that DMS might be an important factor in climate regulation. Known for originating the Gaia hypothesis of life on Earth acting in concert as a complex system like an organism, in 1987 he suggested that by encouraging cloud formation, DMS acted as the Earth’s thermostat and prevented overheating. This became known as the CLAW hypothesis. ‘The oxidation products of DMS, like sulfur dioxide, and other [sulfate] compounds can eventually form new aerosol particles,’ explains Martí Galí, a marine scientist at the Institute of Marine Sciences in Barcelona, Spain. The particles lead to nucleation of water vapour, forming clouds and causing greater reflection of radiation due to the albedo effect (light surfaces reflect more heat than dark surfaces). This could then offset some of the impacts of greenhouse gas warming.
Oxidation state flexibility
The biogeochemical sulfur cycle describes how sulfur in different chemical states moves between living systems, the ocean, land and the atmosphere; the ocean is a major sulfur reservoir, containing large quantities of dissolved sulfate wash-out from gypsum and other minerals. Bacterial species can reduce these to sulfides, which are incorporated into organic compounds. Small sulfur-containing molecules eventually released into the ocean return to the atmosphere as DMS, which is then oxidised and recycled via rainwater.
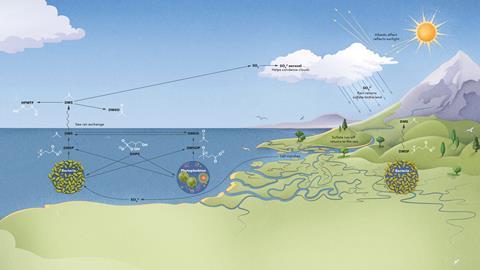
It is the biological part of the sulfur cycle where there is still so much to learn. ‘You always have to face the problem that, if you investigate a biological system, it is per definition small and you [are] looking at [connecting that to] a global element cycling that is per definition enormous… to connect those in an analytically sound way is really challenging,’ says Georg Pohnert, a chemical ecologist from Friedrich Schiller University Jena in Germany.
Sulfur is a constituent of many proteins and cofactors and is present in two of the proteinogenic amino acids, methionine and cysteine. ‘In marine organisms, it’s abundance is comparable to phosphorus in terms of how it accumulates in biomass,’ says Bryndam Durham, a marine microbiologist from the University of Florida. As well as its relative ubiquity, Pohnert explains that its role in biology springs from its chemical versatility, with a wide range of oxidation states from –2 to +6, unlike its fellow group 6 element oxygen. ‘Since you can find it in so many different oxidation states, you have multiple ways of accessing it as a biological entity and you always have multiple ways of processing it. This flexibility makes it a key player in many cellular processes.’
For several decades it’s been known that DMS emitted from the oceans largely comes from the breakdown of the sulfur molecule dimethyl sulfinoproprianate (DMSP), a highly polar molecule containing a positively charged sulfonium ion. DMSP is made by phytoplankton, the photosynthesising microscopic algae found in the ocean surface layer. ‘Some of those organisms produce it in huge quantities, [up to] half molar intracellular concentrations; clearly they put a lot of energy into doing that,’ says Jonathan Todd, a molecular biologist at the University of East Anglia in the UK. When degraded, the molecule is cleaved into DMS, much of which ends up in the atmosphere, and a proprionate fragment which can be metabolised as a carbon source.
But phytoplankton are not the only producers of DMSP. ‘One of the key discoveries from my lab recently is that the hypothesis that DMSP was only made by marine eukaryotic organisms is completely false,’ says Todd. He has found high levels of DMSP and DMS in marshland and coastal sediments linked to DMSP-producing bacteria, estimating there may be as many as 100 million DMSP-producing bacteria per gram of salt marsh sediment – a part of the sulfur cycle previously neglected.
‘We’ve been studying DMSP cycling in surface [coastal] sediment samples and we find the DMSP concentration in sediments is an order of magnitude, or sometimes even two orders of magnitude, higher than what you see in surface seawater.’ Todd says phytoplankton are still the main producers, but his work shows bacteria in mud flats and tidal regions now also need to be factored in as significant players.
Missing pathways
One question Todd and others are asking is why are so many organisms producing DMSP? What is its use?
‘There are loads of hypotheses that you could read all day, and you’d find 101 different suggestions,’ answers Todd. ‘The common perception is that it’s produced by marine eukaryotic organisms as an anti-stress compound.’ In 2017 his lab identified the key gene responsible for its biosynthesis in marine bacteria and also noted the gene was upregulated in environments where salinity was increased, temperatures lowered or nitrogen concentrations limited.
We found that there was huge biodiversity in the ways organisms generate DMS
DMSP seems to have a role in osmoregulation for some phytoplankton, making use of its zwitterionic properties. For example, diatoms are algae uniquely enclosed by a transparent silica cell wall, meaning they are unable to regulate their concentration by changing size. What they do instead is use the charged DMSP molecule, explains Pohnert. ‘They produce salts by a biosynthetic processes… and then they can also regulate it down again.’ Todd says DMSP and its products DMS and propionic acid may also be produced as signalling molecules by a variety of microbes; for example, propionic acid can be toxic to some organisms but DMS is also a chemo-attractant.
A clue to the diversity of DMSP use also comes from the work Todd and collaborators have done on identifying the enzymatic pathways that break it down. After identifying the first DMSP lyase enzyme they thought it ‘would be the end of the story’. But they ultimately found eight unique enzymes in algae and bacteria, all from distinct protein families with unique chemical pathways. ‘We found that there was huge biodiversity in the ways in which microorganisms and higher organisms degrade DMSP to generate DMS.’
It has also recently become apparent that a diverse ocean ecosytem has developed that not only makes but consumes DMSP and other organo-sulfur compounds. ‘We generally think of the marine environments being awash of nutrients, but actually the open ocean is quite sparse and DMSP is a key [nutrient]. A wide range of microorganisms import DMSP and metabolise it as a source of carbon and sulfur for energy,’ explains Todd. According to Durham, these DMSP feeders make part of a series of cooperative interactions. ‘Bacteria that can use DMSP are thought of as being beneficial to phytoplankton [that make DMSP], they’ll make vitamins, signalling molecules, hormones, all sorts of things.’ Williams compares this sulfur ecosystem to a coral reef, with organo-sufur compounds passed from one organism to another, like currency.
In 2018 Pohnert discovered another missing pathway or ‘shortcut’ in the marine sulfur cycle, with the existence of dimethylsulfonium propionate (DMSOP) found in all ocean samples from the Arctic to the Mediterranean. ‘There is an entire family of compounds structurally related to DMSP and we were quite surprised to even find a sulfoxonium compound, which is chemically very unusual.’ DMSOP is also a zwitterion that can be broken down by enzymes to two non-charged units, but with sulfur in a higher oxidation state than DMSP.
‘We can’t explain why both compounds are required,’ says Pohnert, but he suggests by converting DMSP into DMSOP, some species of algae and bacteria are able to survive an increase in reactive oxygen species that they may encounter in moving through a quickly changing ocean – essentially an internal detoxification mechanism. ‘It’s really just another fine-tuning of their ability to adapt to their environment,’ says Pohnert, who suspects DMSOP production could also be linked to ageing, where the oxidative balance in organisms can become distorted; ‘That’s an idea that we are following up now.’
Mind the gaps
Pohnert has filled in the gaps of this part of the sulfur cycle. Like DMSP, there are bacteria able to metabolise DMSOP, forming dimethyl sulfoxide (DMSO), which can be itself be converted to DMS or taken up by other bacteria.
Sulfonated molecules are not something that anybody had ever been thinking about in marine systems
‘We really thought that [DMSP] was the main thing in terms of sulfur cycling [and] if we could quantify the DMSP metabolism, we’d pretty much have the sulfur budget figured out.’ says Durham, but in 2019 she also discovered things to be more complicated when DMSP was absent from some of her marine samples. Then working in the lab of Virginia Armbrust from the University of Washington in Seattle, US, they discovered communities of phytoplankton and bacteria instead making and metabolising sulfonates, molecules containing the functional group R-SO3⁻, sulfur in a +5 oxidation state.
‘We were shocked. Sulfonated molecules are not something that anybody had ever been thinking about in marine systems,’ says Durham. But they are abundant in terrestrial systems which provided a clue. Durham found genes present in these marine organisms that probably share a common origin with those controlling the comparable pathways in soil bacteria.
Sulfoquinovose is the most important molecule you’ve never heard of
In particular she found 2,3-dihydroxypropane-1-sulfonate (DHPS) produced in diatoms in millimolar concentrations which was metabolised by bacteria as a source of carbon and sulfur. ‘What we understand about sulfonates lags behind how we understand DMSP,’ says Durham. Why they are made is not clear, but one suggestion from Durham is that it may be a way of regulating photosynthesis – they know from organisms cultured in the lab that DHPS is only produced during the day. ‘If there’s a lot of incoming light, phytoplankton don’t have sunscreen, they just have to deal with it. So sulfonate assimilation is energy intensive and might be a good way to dump excess electrons… that’s what we’re imagining.’
The discovery of DHPS and its link to photosynthesis rang some bells with Williams, a carbohydrate chemist who studies glycosis pathways and the similar biological processes for breaking down the sulfonated monosaccharide sulfoquinovose. ‘I call sulfoquinovose the most important molecule you’ve never heard of,’ says Williams. ‘It looks like glucose except it’s got a carbon–sulfur bond.’ It is estimated to make up around 50% of all organosulfur molecules (the remaining largely made up of cystine and methionine).
Sugarcoating the problem
‘Almost every single photosynthetic organism, whether it be a cyanobacteria, algae, diatoms, plants or moss, produces sulfoquinovose,’ says Williams. Its ubiquity is explained by its role in photosynthesis, being part of the membranes that surround the compartments known as thulakoids, inside chloroplasts where the photochemical reaction takes place. Instead of exclusively phospholipids these membranes contain the glyco-lipid, sulfoquinovosyl diacylglycerols (SQDG).
Williams has elucidated the enzymatic pathways by which niche soil bacteria are able to harvest and break down sulfoquinovose from plant matter. ‘[In] every gram of soil that you can find anywhere there’ll be a bug that has a latent [enzymatic] pathway, waiting to get lucky and get a little bit of this sulfuoquinovose.’ But it turns out that no single organism can break down sulfoquinovose. Instead, explains Williams, they tend to spit out a sulfur fragment which is passed on to other organisms. One of those sulfur molecules is DHPS, the sulfonate observed for the first time in the oceans by Durham in 2019. While there is no clear evidence yet, Williams suggests sulfoquinovose from photoplankton could be the source of ocean DHPS. ‘Maybe that’s where it’s coming from,’ he says – but concedes no one really knows yet.
Sulfoquinovose is also one of the ways in which humans interact with the sulfur cycle. Our gut microbiome includes the Firmicutes bacterial family, which metabolise sulfoquinovose from the food we eat. ‘You’re not eating a huge amount. We did a calculation that if you’re on the Popeye diet, eating a lot of spinach, you might have a few hundred milligrams of sulfoquinavose a day,’ says Williams. But this is enough to support this niche bacteria. The process ultimately produces an additional source of hydrogen sulfide, which will be returned to the atmosphere to be recycled.
No single organism can break down sulfoquinovose – they tend to spit out a sulfur fragment which is passed on to other organisms
For climate modellers, understanding the sulfur cycle and how it has responded to climate change is important for more accurate climate prediction. While sulfur released from fossil fuels has doubled environmental levels since the industrial revolution and is still the predominant source, DMS from the oceans accounts for a not insubstantial third of total atmospheric sulfur. Anthropogenic sulfur is the cause of acid rain, which can significantly degrade ecosystems. In the US and Europe, legislation has all but fixed this problem, but it still remains a concern in other parts of the world.
There is still uncertainty surrounding the impact of sulfur compounds. In his 2006 book The Revenge of Gaia, Lovelock expanded on his previous ideas and suggested that global warming was leading to a decrease in the DMS-producing ocean biomass, reducing the potential positive feedback effects he had earlier predicted and perhaps creating a spiralling effect.
Whether this is the case is not yet clear. ‘Currently the models [of DMS production] have some deficiencies,’ says Gali. He points to the four state-of-the-art climate models published in 2021 by the World Climate Research Programme and underpinning the IPCC’s sixth assessment report. ‘[The] four of them have alternative representation of DMS emissions… two models predicted an increase during the next century, the other two predicted a decrease.’ Although the effect may ultimately be small, Gali says it could still have an impact and there is now a strong imperative to more accurately model DMS production. Todd agrees and adds that the contribution of DMSP from other environments, such as the marshlands he has studied, also need to be factored in.
There are also new discoveries in other parts of the cycle. A 2020 study from the US National Oceanic and Atmospheric Administration, for example, identified that 30% of DMS is oxidised to hydroperoxymethyl thioformate, identified through airborne observation. It’s impact on aerosol formation and cloud condensation is yet to be investigated.
On global scale, Gali has started the job of measuring and modelling DMS. In 2018 he created an algorithm to estimate marine DMS levels using remote sensing satellite data. For example, he calculates phytoplankton biomass levels based on optical measurements that can estimate the amount of chlorophyl present from analysing colour intensity. But he says what is now really important is to be able to accurately establish global rates of change, and this will need a lot more work.
He is now hoping to create a database of global DMS production and consumption rates, through the Special Committee on Oceanic Research, an NGO managing international marine research. This would ultimately provide data for more accurate climate modelling. It will require a much more detailed analysis than currently exists; for example, being able to distinguish low and high DSM producers and fully understand how other microbes contribute to the consumption and production of interrelated organosulfur compounds. ‘You have to represent all these processes in your models to get the sulfur concentration right,’ says Gali. ‘It’s quite complex.’ And given recent discoveries there may very well be parts of the sulfur cycle still to uncover. But, as Pohnert concludes, to really pinpoint its impact on climate, we will need to improve our understanding of the sulfur cycle and it will need to be a multidisciplinary effort; ‘we need an interplay between modelling, microbiology and chemistry’.
Rachel Brazil is a science writer based in London, UK












1 Reader's comment