The Restek Biphenyl LC column trailblazed a new approach to LC separations. Today, as copycat columns continue to come out, Restek remains committed to innovation, not imitation. Chemistry World finds out about innovation behind Biphenyl and how the Biphenyl LC column can help simplify your separations and advance your science today.
Twenty years ago, Restek was the first to bring chromatographers the pioneering Biphenyl LC column. Ideal for bioanalytical testing applications, such as drug and metabolite analyses, its exceptionally unique selectivity made it possible to achieve better separations than were possible on traditional C18 columns or other phenyl chemistries.
From small idea to big dream
In 2005, a team within the LC development group began working on a novel phenyl chemistry centred around the biphenyl molecule. The dream was to develop a new stationary phase, one with a unique selectivity compared to other LC columns. Achieving this would provide labs with a novel tool to help with their LC analysis—an exciting prospect for any team. Little did they know how big an impact they were about to make on the entire chromatography industry.
The advantages found in using the Biphenyl column are certainly the high price-quality ratio, the durability of the column, in terms of numbers of injections, as well as the analytical reproducibility
Cedam Italia, Italy
“We knew it was different, and different is good.”
Early results on prototype columns instantly demonstrated the Biphenyl LC column’s truly unique selectivity. It proved to be especially useful for analytes containing electron-withdrawing functionalities and could also be easily modified from analysis to analysis with varying mobile phase conditions. Additionally, it was particularly adept at separating compounds that are hard to resolve on C18 columns. Randy Romesberg, one of the scientists who helped develop this phase, stated “We knew it was different, and different is good.”
The team had achieved a first in the industry—the launch of the first LC column with a Biphenyl stationary phase.
The separation of aflatoxin B1, G1, B2, G2, OTA, zearalenone, deoxynivalenol, T2, H-T2, fumonisin B1 and B2 after CrossTOX cleanup in high-throughput mycotoxin analysis was very good. The shorter conditioning times were also very beneficial. I can certainly also imagine the Inert Biphenyl column as an alternative for other applications.
LCTech GMbH, Germany
The right phase at the right time
Long regarded as having superior chromatographic performance in drugs of abuse analysis, Restek’s Biphenyl LC column became an essential tool at the height of the U.S. opioid crisis in 2011. The Big Pain Application demonstrates its incredible performance when separating 231 compounds, 40+ isobars, 10 drug classes, and 22 ESI- compounds in 10 minutes. The Biphenyl’s incredible separating power and superiority in pain management applications made it a clear choice for clinical testing laboratories.
We were able to achieve a faster run time than our previous column, with great separation between analytes, especially with opiates. We have been very happy with the column and currently run it for all four of our Triple Quad 4500s.
Oklahoma Pain Center, US
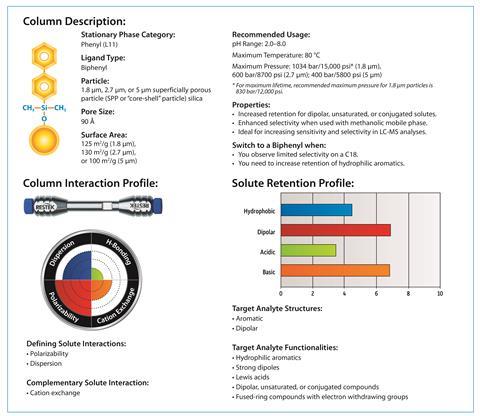
Case study: Putting Biphenyl into practice
Comprehensive mycotoxin analysis: simultaneous determination of Alternaria toxins, ergot alkaloid epimers, and other major mycotoxins in various food matrices by LC-MS/MS
Mycotoxins, which are produced by a diverse range of fungi, are widespread food contaminants that have significant impacts on both human health and global economics. The prevalence and co-occurrence of different classes of mycotoxins vary greatly among geographic location and crop types, which has resulted in a complex international landscape of regulations and maximum residue concentrations. A simple method that allows simultaneous determination of multiple mycotoxin classes in different food matrices would greatly improve test lab productivity, but comprehensive methods are difficult to develop due to the wide range of chemical properties exhibited by mycotoxins from different fungal species.
A simplified sample preparation procedure and a reliable LC-MS/MS analytical method were developed for the simultaneous analysis of 37 regulated and emerging mycotoxins, including five Alternaria toxins, six major ergot alkaloids and their corresponding epimers, and priority mycotoxins from other classes. Four different food matrices (wheat baby cereal, peanut, tomato puree, and blended flour) were evaluated to demonstrate the applicability of this method to a wide range of food types.
For multi-mycotoxin analysis, one of the most significant challenges is combining two emerging groups – Alternaria toxins and ergot alkaloids – into methods for other classes of mycotoxins. The most important Alternaria toxins are altenuene, alternariol, alternariol monomethylether, tentoxin, and tenuazonic acid (TeA), which are frequently detected in cereal- and fruit-based food products. Tenuazonic acid is typically present at much higher concentrations than other Alternaria toxins, and several studies have shown that high pH conditions are necessary to obtain acceptable peak shape for it when using a C18 column. Similarly, the most important ergot alkaloids (ergocornine, ergocristine, ergocryptine, ergometrine, ergosine, and ergotamine, together with their isomeric -inine epimers) require high pH LC conditions to achieve full separation on a C18 column. Use of high pH conditions is stressful for LC columns and not suitable for the chromatographic analysis of other classes of mycotoxins, so these conditions are not an ideal basis for comprehensive methods.
Another factor that complicates any comprehensive method is the need to mitigate matrix suppression or enhancement. Using cleanup procedures on sample extracts to reduce such matrix effects is common, but due to the variation in mycotoxin chemical characteristics, it is unlikely that a single cleanup protocol would produce proper recoveries and accurate quantification for all types of mycotoxins. The use of stable isotopes as internal standards is the most direct way to correct matrix effects but, due to the lack of isotopically labelled compounds for most of mycotoxins, it is not feasible for comprehensive mycotoxin analysis. Matrix-matched external standard calibration is a more suitable approach for multi-mycotoxin analysis, but it is dependent on finding a similar food matrix to be used as the blank matrix.
In this study, we successfully developed a comprehensive quantitative method that is suitable for the simultaneous analysis of Alternaria toxins, ergot alkaloids and their epimers, and other major mycotoxins produced by Aspergillus, Fusarium, and Penicillium fungi. A simple sample preparation procedure was paired with fast, acidic LC conditions for accurate and robust analysis of 37 regulated and emerging mycotoxins. This method was developed on a Raptor Biphenyl column instead of a C18 column so desirable acidic conditions could be used. Four food matrices (wheat baby cereal, peanut, tomato puree, and blended flour) were used to demonstrate the applicability of this method to a wide range of food types. Matrix-matched calibration was used to offset matrix effects, and the method was demonstrated to be effective in terms of linearity, specificity, accuracy, precision, and adaptability.
The primary reported drawback to mycotoxin analysis on C18 columns is that high pH conditions are required for Alternaria toxins and ergot alkaloids, which prohibits adding them to existing methods for other mycotoxins. Indeed, our preliminary experiments confirmed that when using a C18 column under acidic conditions, tenuazonic acid peak shape was too poor for integration and the ergot alkaloid epimers could not all be separated. In contrast, these emerging mycotoxins can be separated adequately and with satisfactory peak shapes under standard acidic conditions on a Raptor Biphenyl column, allowing for the development of a higher productivity combined method.
Simultaneous analysis of 37 mycotoxins was achieved in a fast, 11-minute total cycle time run on a Raptor Biphenyl column under acidic conditions. Importantly, all epimer pairs of ergot alkaloids were chromatographically separated for definitive and accurate quantification. Epimer stability was evaluated during method development and no conversion was observed under the low pH conditions used here. In addition, quantifiable peak shape was obtained for tenuazonic acid, although method development work demonstrated that when a new column was installed it had to be rinsed and maintained under mobile phase overnight to ensure an acceptable peak shape for this challenging compound.
The workflow in this study provides a unique solution for mycotoxin analysis that allows simultaneous determination of Alternaria toxins and ergot alkaloid epimers along with other major mycotoxins produced by Aspergillus, Fusarium, and Penicillium fungi. The method established here combines a simple sample preparation and a fast, 11-minute analysis on a Raptor Biphenyl column under low pH conditions. Good chromatographic results were obtained—including complete separation of all six ergot alkaloids and their epimers—and the method has been demonstrated to be rugged, accurate, and precise for quantitative determination of mycotoxins in a wide variety of food products. Use of a simplified sample preparation procedure and concurrent analysis under low pH conditions provides an important opportunity for labs to improve productivity through more comprehensive methods.
Find the Biphenyl column for you now!
Additional information
Case study is an extract from SH Liang et al. J AOAC Int, 2023. DOI: 10.1093/jaoacint/qsac138