Chemists have used 3D printing to produce plastic capsules that store and isolate chemicals for organic synthesis. Soluble in common organic solvents, the capsules release compounds directly within a reaction mixture, offering a safer system for handling volatile, toxic and flammable reagents in the lab.
Synthetic chemists often work with hazardous chemicals. For example, many organometallic reagents are corrosive, some acetylides are explosive, ethers are flammable and many aromatic amines are carcinogenic.
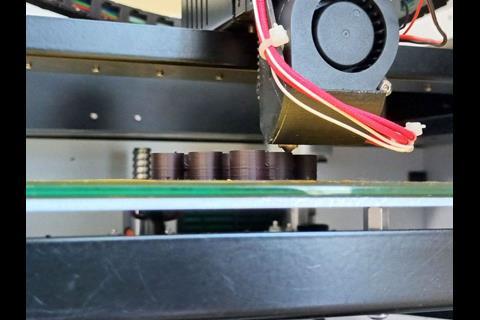
Last year, researchers in the UK showed how encapsulating organolithium compounds in organogels meant these extremely reactive reagents could be handled in air. Now, researchers led by Valentine Ananikov, from the Zelinsky Institute of Organic Chemistry in Russia, have designed 3D-printed thermoplastic capsules that can hold a variety of reagents. When added to a solvent, the capsules dissolve within minutes, releasing their contents to initiate a reaction. The team proved how the capsules could be made from different polymers, including high impact polystyrene, polyvinyl alcohol and polylactide, which rendered them compatible with solvents such as ethyl acetate, 2-methyltetrahydrofuran, acetonitrile and water.
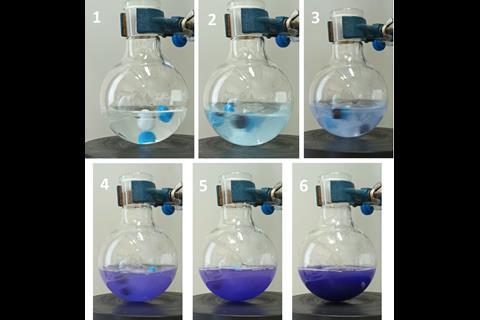
The team confined air-sensitive carboxylic acid chlorides, volatile amines and triethylamine in pre-measured amounts to test the capsules on an amine N-acylation and found it ‘cuts down preparation time drastically’, explains Ananikov. ‘Handling chemicals for a reaction involving several components [such as N-acylation] typically requires a minimum of 15 minutes’ for manual dosing, weighing and transferring to a reaction vessel, whereas using the capsules took 15 seconds. After the reaction, they evaporated the solvent and added ether to precipitate the polylactide plastic while leaving the amide product dissolved. Filtering and drying the plastic turned it back into plastic filament for 3D printing.
Using different polymers in a single capsule makes it possible to dissolve part of it but recycle the rest. For example, they tested making the capsules ‘in a way where only the top dissolves. Actually, that’s pretty cool,’ comments Stellios Arseniyadis, an expert in synthetic methods from Queen Mary University of London in the UK. In this case, there was no effect on product yield, but there is a risk that compounds adsorb to the recycled component and contaminate future reactions.
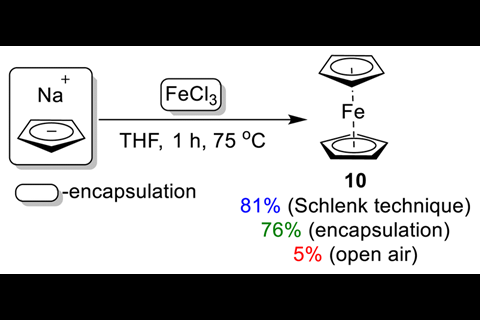
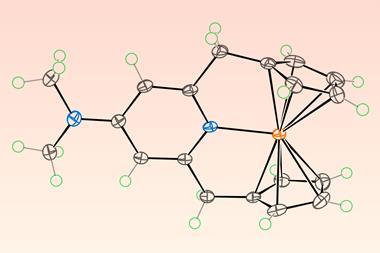
Ananikov’s team also used their system to synthesise ferrocene. They confined sodium cyclopentadienide and anhydrous iron (iii) chloride within high impact polystyrene capsules. Handling air-sensitive chemicals like sodium cyclopentadienide often necessitates a Schlenk line, so encapsulating it ‘makes life a lot simpler in terms of your workflow and reduces the risk to people in the laboratory,’ says Stephen Hilton, an expert in 3D printing in chemical synthesis from University College London in the UK. Their reaction yielded ferrocene with 76% efficiency, compared to 81% with a Schlenk line, which Ananikov describes as a benchmark for ‘further refinement, especially in protecting sensitive chemicals within the capsules’.
Ananikov and co-workers are currently exploring other polymer materials, including those which are biodegradable, to make the system compatible with other chemicals and reactions. And although the concept is still in a preliminary stage, Ananikov says its ‘real power comes from commercialisation’. Combining 3D printing with automation could, he says, ‘very well be the dawn of a new era in chemical synthesis, where safety, efficiency and sustainability are not just aspirations but realities.’
References
A N Lebedev et al, Green Chem., 2024, DOI: 10.1039/d3gc04064j















No comments yet