Metal–organic frameworks (MOFs) could offer a safer method for handling fluorinated gases in the lab, US researchers have found. It is hoped the technique will assist the synthesis of fluorinated compounds required in medicinal chemistry, agriculture and biomedical imaging.
‘The problem is that these fluorinated building blocks are all gases at room temperature, and most are environmentally destructive, toxic and/or flammable,’ explains lead researcher Kaitlyn Keasler, from Cornell University in New York, US. ‘Safely working with gaseous reagents requires the use of specialised equipment, or a means to generate the gas in situ, neither of which is amenable to reaction development,’ she adds.
The researchers set out to find a porous material with high gas sorption capacity that would interact strongly with a range of fluorinated gases to prevent leakage, while remaining stable for extended periods.
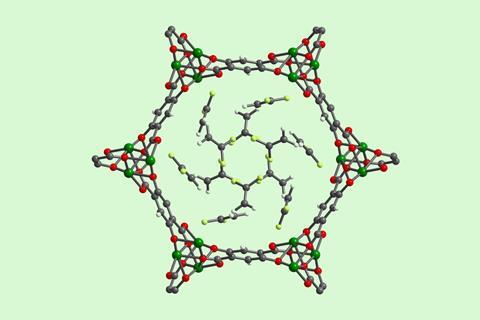
They started by studying the uptake of vinylidene fluoride (VDF) in 12 representative open-metal-site MOFs. The team found that Mg2(dobdc) was an ideal framework for the storage of fluorinated gases because of its high capacity and strong interaction with multiple gases of synthetic interest. The MOF also retained its crystallinity and porosity after a week on a lab benchtop at room temperature and was stable in air at 120°C for 24 hours.
‘We demonstrated that the optimal MOF for reversibly storing fluorinated gases is one that possesses coordinatively unsaturated Mg2 centres likely due to the hard–soft acid–base interaction of hard fluorine atoms with hard magnesium centres,’ explains Keasler. ‘In order to accelerate reaction development using the optimal MOF, we developed a new high concentration aqueous synthesis that enables Mg2(dobdc) to be prepared on 100g scale.’ Once the gas–MOF reagent is dropped into a solvent the structure breaks down releasing the fluorinated compound.
Finally, they explored the stability of the gas–MOF reagents and found that multi-gram samples of gas–MOF reagents could be prepared and stored in air for up to a week without compromising their performance. They also found that embedding the gas–MOF agents in wax capsules enabled their stable storage in air at room temperature for up to two months. The wax capsules can then be easily broken open by sonication of the reaction mixture, releasing the fluorinated reagent into solution.
Riya Halder, a PhD candidate in the Ritter Group at Max-Planck-Institut für Kohlenforschung, says the method was an attempt to ‘tame the tiger of chemistry’. ‘Sometimes, while working with highly reactive gases, we struggle to control the regioselectivity and stereoselectivity outcomes of the reactions, however, in this case, the gases can be released in a controlled way by sonication,’ she says. ‘Another big advantage of the method is that, after use, the MOFs can be filtered off, recovered and recycled.’
David O’Hagan, an organofluorine chemist at the University of St Andrew’s, says the technique could open up ‘many prospects’ to access high value fine chemicals and new pharmaceutical candidates. ‘Volatile fluorinated gases are produced on a large scale industrially and therefore they are cheap, but they are not available to non-specialists, even in solution – there are handling and exposure issues to consider. If wax sealed MOFs, loaded with readily quantifiable millimolar doses can become easily available to researchers, then a lot of new chemistry would follow.’
References
K Keasler et al, Science, 2023, 381, 6665 (DOI: 10.1126/science.adg8835)














No comments yet