Ultrasound has been used to print polymers deep inside the body without damaging surrounding tissue. The protocol, which was demonstrated in animals, could have multiple applications such as drug delivery and wound healing.
3D printing has had a big impact on medicine, allowing the creation of patient-specific implants and tissue replacements. At present, however, these must be printed externally before being surgically implanted. It would therefore be highly desirable if doctors could inject biocompatible inks before curing them at the desired point. The opportunity for light-triggered polymerisation is limited, however, because even in tissue’s near-infrared ‘transparency window’, radiation is attenuated so quickly that printing more than a few millimetres beneath the skin is impossible.
Focused ultrasound presents a promising alternative as it can penetrate deep into tissue. In 2023, researchers at Harvard University used it to induce sonothermal polymerisation of poly(ethylene glycol) diacrylate. Unfortunately, the temperatures generated were 60–70°C.1 ‘I don’t know about you, but I’d rather not be made medium rare,’ says bioengineer Mark Skylar-Scott at Stanford University.
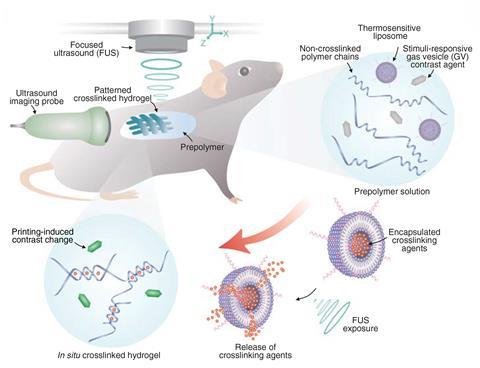
Working independently of the Harvard researchers, Wei Gao at Stanford University and colleagues have developed a technique in which polymer crosslinking agents are encapsulated into liposomes that break down between 37°C and 42°C.2 These are mixed into inks comprising biopolymers and ultrasound-sensitive contrast agents and injected into an organism. These can be injected and rapidly crosslinked using a beam of focused ultrasound. ‘These bio-inks are not like water – they’re more viscous, so they cannot easily flow anywhere in a short period of time,’ explains Gao.
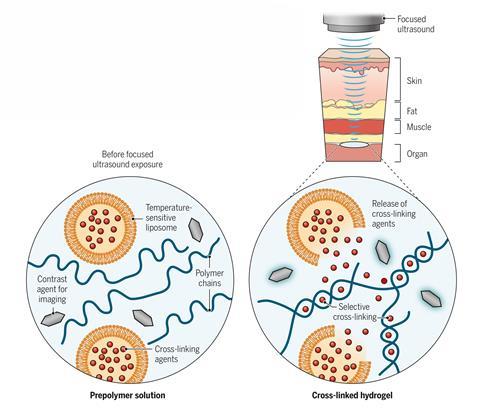
The researchers formed multiple different polymers, including an ionically crosslinked alginate using calcium chloride-loaded liposomes, a free radical crosslinked polymer by loading the liposomes with tetramethylethylenediamine and an oxidatively crosslinked adhesive hydrogel that could seal internal wounds using liposomes loaded with sodium periodate.
By adding other materials to the inks, the researchers incorporated various additional functionalities to the materials. Adding the anti-cancer drug doxorubicin yielded a drug-loaded hydrogel. By printing this actually inside a mouse bladder, they showed that they could localise the drug to the site of a tumour. In vitro, they showed that using such a hydrogel led to markedly greater cancer cell death than free drug administration. The researchers also showed that adding carbon nanotubes or silver nanowires produced conductive polymers suitable for implantable bioelectronics.
There was no sign of toxicity from experiments in mice and rabbits. Crosslinked polymers persisted inside the body, but uncrosslinked inks were cleared within seven days.
An important next step for the technology, says Gao, will be to target the focused ultrasound towards dynamic tissue. ‘If you have a vigorously beating heart, for example, we want a real-time, integrated algorithm to do the imaging and in vivo printing,’ he says.
‘I think it’s an excellent advance in the field of in situ bio-printing,’ says Skylar-Scott, who was not involved in the work. He suggests that, in future, it could be possible to create multi-material tissue scaffolds inside the body by injecting different bio-inks at different times. He does note that the axial resolution at present is only around 2mm, but suspects this will probably be improved in future. ‘I think it opens up a lot of different avenues to explore.’
References
1 X Kuang et al, Science, 2023, 382, 1148 (DOI: 10.1126/science.adi1563)
2 E Davoodi et al, Science, 2025, 388, 616 (DOI: 10.1126/science.adt0293)














No comments yet