A small molecule’s big moment
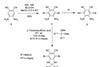
Substituted benzene has the largest dipole ever observed in a neutral molecule
By Katrina Krämer2016-02-08T00:00:00

Source: Wiley
Substituted benzene has the largest dipole ever observed in a neutral molecule
Site powered by Webvision Cloud