Chemists prepare ‘twistacenes’ with torsion angles of up to 170º
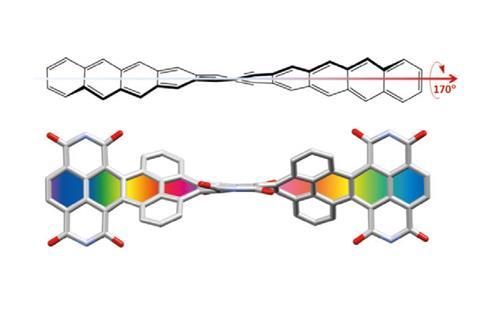
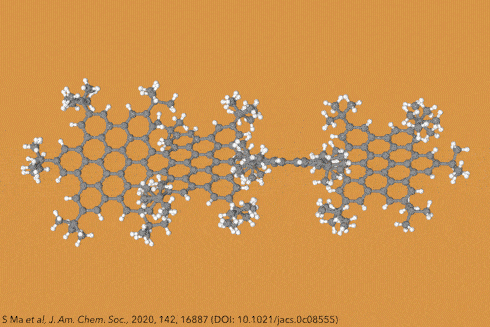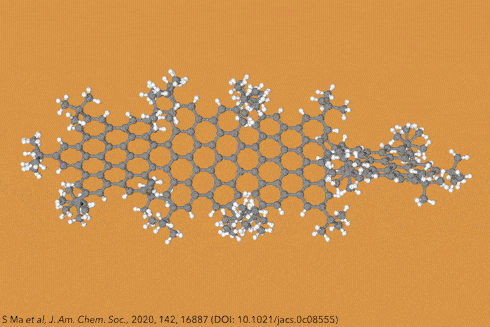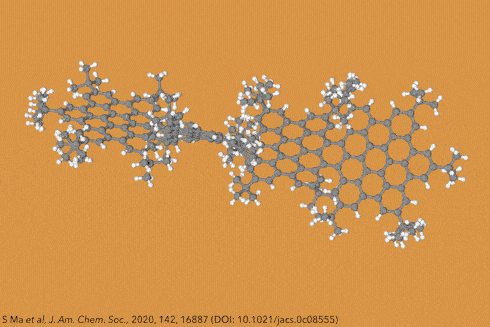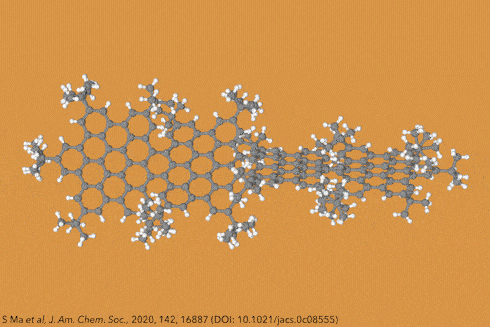
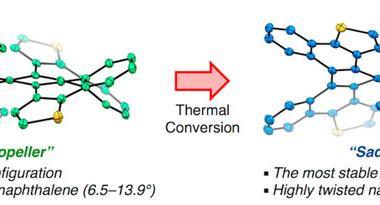
Twisting acenes by introducing sterically crowded groups has produced a family of molecules dubbed twistacenes. For a long time, these molecules remained practically unexplored, mostly because their synthesis is extremely challenging. Now, a team of chemists report the synthesis of decatwistacene, which is the longest of its kind.1 The molecule also exhibits a record torsion angle of 170°, surpassing the 144° of highly twisted pentacene.2
Despite being so hard to prepare, twistacenes have always interested chemists. Due to their unusual topology, these molecules show better solubility in organic solvents and air stability than ‘naked’ acenes. Moreover, twists in the π system result in chiral molecules, making twistacenes attractive for non-linear optics.
Years ago, it was discovered that imide groups could stabilise acenes,3 and it was this strategy that was used to prepare decatwistacene. Combining the introduction of these hindering groups with palladium catalysed cross-coupling and C–H activation, researchers simply obtained decatwistacene, and also hexatwistacene. Both molecules are very stable solids – dark purple and red, respectively – and can be crystallised and characterised using x-ray diffraction.
Using both experimental and computational techniques, the chemists examined the optoelectronic properties of hexa- and decatwistacene. Their results suggest that twistacenes are potential n-type semiconductors with excellent stability. These imide-substituted twistacenes pave the way for stable, easy to prepare chiral graphene nanoribbons with promising applications in electronics.
References
1 W Fan et al, Angew. Chem., Int. Ed., 2017, DOI: 10.1002/anie.201709342
2 J Lu et al, J. Am. Chem. Soc., 2004, 126, 11168 (DOI: 10.1021/ja046576w)
3 W Yue et al, J. Am. Chem. Soc., 2011, 133, 18054 (DOI: 10.1021/ja207630a)













No comments yet