A new algorithm based on physical principles can design proteins that bind cancer drugs to potentially head off an overdose or allergic reaction. The researchers believe that the algorithm could potentially be more effective than deep learning approaches for designing proteins with unfamiliar requirements. This could make it useful for producing antidotes or delivery vehicles for drugs.1
Protein structure prediction has advanced hugely in recent years because of deep learning algorithms such as Deepmind’s AlphaFold and the Institute for Protein Design’s RoseTTAFold. These scour known structures in the Protein Data Bank and learn to predict how unknown structures will fold. They work purely using probability, however: energy does not appear explicitly in their calculations. ‘Deep learning could be learning physical principles,’ says Nicholas Polizzi of Harvard Medical School in Massachusetts, US, ‘and the way to test that is to design things with it that are totally outside the distribution of the training data. If it’s successful, you know it really did learn something and is able to generalise. So far, I haven’t really seen that with deep learning in protein design.’
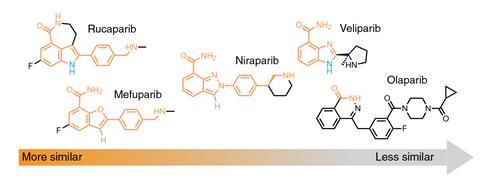
One test is designing a protein that can bind to a small molecule not found in the protein data bank. In 2020, Polizzi and William DeGrado at the University of California, San Francisco developed a physics-based computer algorithm and used it to design two proteins that bound the blood-thinner apixaban.2 Polizzi and researchers in DeGrado’s group led by Lei Lu have now developed an extended algorithm and used it to design proteins to bind a series of novel cancer drugs called poly(ADP-ribose) polymerase–1 inhibitors (PARPi ). Protein—drug conjugates such as these could prove invaluable for mitigating the effects of overdose or allergic reactions to drugs. They could also potentially be used to produce ‘smart’ delivery vehicles if proteins could be designed that are broken down at the target site to release the drug they bind. Further into the future, the understanding of small molecule–protein binding might be used to design drugs to target rogue proteins.
Proteins are too large for full electronic structure theory calculations, but the researchers incorporate rules governing the energetic stability of a configuration. Polar groups are energetically inclined to form hydrogen bonds to water, for example, so isolating a polar group in the interior of a protein carries an energetic cost. ‘If you remove hydrogen bonds from water by dragging material into the interior of a protein, you’d better replace those hydrogen bonds with hydrogen bonds to the protein,’ says Polizzi.
Using considerations such as these, the researchers designed proteins to bind the PARPi drug rucaparib and synthesised the most promising candidates. Two of these bound rucaparib with nanomolar-range dissociation constants. The researchers also tested the proteins’ binding affinities for other PARPi. The dissociation constant increased as expected as the structure diverged.
Polizzi says it was not obvious that the type of protein they were using would prove a good candidate. ‘It’s another example of extrapolation, where it would be very difficult to train a deep learning algorithm because there’s not a lot of examples of four-helix bundles binding small molecules,’ says Polizzi. The Institute for Protein Design very recently unveiled an updated version of their algorithm called RoseTTAFold All-Atom that incorporated molecules beyond amino acids, but Polizzi believes that ‘right now … deep learning models have not accomplished what Lei has accomplished’.
Biochemist Anum Glasgow at Columbia University in the US believes that RoseTTAFold All-Atom and the new model from Lei and colleagues collectively open doors for protein designers. ‘Naturally evolved proteins can do really interesting and sophisticated functions by having, for example, water-mediated hydrogen bonds or really subtle conformational changes … those things are still kind of beyond our capabilities, although they’re really exciting areas for the field,’ she says. ‘I can’t say which method is better for an arbitrary small molecule target – I think as protein engineers we would try both strategies and probably combine them.’
References
1 L Lu et al, Science, 2024, 384, 106 DOI: 10.1126/science.adl5364
2 NF Polizzi and WF Degrado, Science, 2020, 369, 1227 (DOI: 10.1126/science.abb8330)













No comments yet