Fully automated reactor can carry out multistep reactions using both batch and flow chemistry
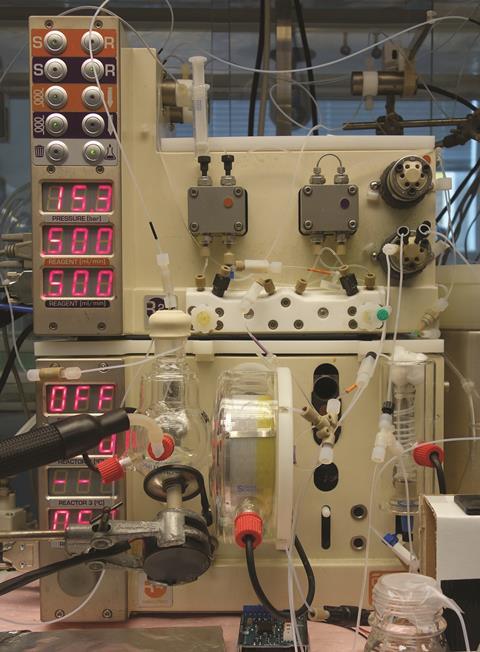
UK researchers have developed an automated reactor that unites two rival systems – batch and flow chemistry.
Batch processes are reactions carried out in single vessels. In multistep syntheses, batch reactions usually require significant manual labour by a researcher, as the reaction products from each step must be processed separately. In flow processes on the other hand, a researcher sets up a reaction sequence in a flowing stream and allows it to run continuously under controlled conditions. This can reduce waste and save time and money. However, not all reactions are amenable to flow chemistry, and many multistep organic syntheses require some batch and some flow steps.
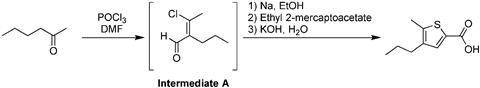
Now, Steve Ley and Daniel Fitzpatrick at the University of Cambridge have developed an automated system that can carry out multistep syntheses, including post-reaction processing, using both batch and flow techniques. To test their system, the team prepared 5-methyl-4-propylthiophene-2-carboxylic acid from 2-hexanone – a complex, multistep procedure with several workup steps such as switching solvents. ‘The system is designed to provide a more holistic understanding of synthesis practice, so that transition across boundaries moving from discovery to larger scale processes will be minimised,’ comments Ley.
Ley’s system, which is based on a commercially available modular flow chemistry reactor, allows researchers to choose between batch and flow methods at each step without needing to change pre- or post-reaction processes. Once the reaction sequence is set up, the entire synthesis runs in one continuous phase, managed by only one researcher. The system works at temperatures ranging from –70 to +150ºC and even at high pressure.
Andreas Kirschning, an expert on microreactor technology at the Leibniz University of Hannover, Germany, rates the work highly. ‘What is usually regarded to represent two distinct synthetic philosophies has been turned upside down here,’ he says. ‘It is always remarkable to see how Steve Ley and his group push the frontiers of synthetic technologies forward and try the unthinkable in a very original and creative manner.’
‘The control of combined multistep flow/batch sequences by an automated platform will surely be taken up by other researchers enabling novel, advanced target synthesis,’ adds Thomas Wirth, an expert on microreactor technology at Cardiff University, UK.
With this automated reactor, organic chemists will need to carry out fewer time-consuming lab tasks. Ley has high hopes for the future of this technology. ‘As with all new things, the system will have to evolve to increase versatility and autonomy, but with the availability of new microprocessor control chips and the inventive spirit of the chemist, this will happen,’ he concludes.
References
This article is free to access until 25 November 2016
D E Fitzpatrick and S V Ley, React. Chem. Eng., 2016, DOI: 10.1039/C6RE00160B











No comments yet