Micromixing enables protecting-group-free synthesis of organolithiums
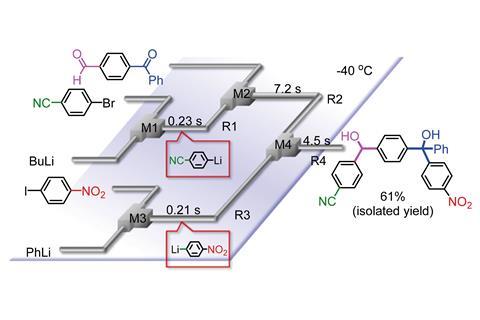
Researchers in Japan have used fast micromixing in a flow microreactor to synthesise highly functionalised organic molecules without using protecting groups.
Organolithium reagents are common in organic synthesis, but their aggressive nature makes them hazardous to use and often promotes side reactions that generate unwanted byproducts. Chemists can synthesise them using protecting groups – modifications added to a molecule to prevent its highly reactive part from reacting while another part of the molecule reacts instead. However, these syntheses require multiple steps for adding and removing the protecting group, which can be costly and time-consuming.
Protecting-group-free synthesis has generated a lot of attention in recent years because it avoids additional reaction steps. Scientists have used flow reactors for selective lithiations in the past, but these have been limited to difunctional products, with further chemoselective functionalisation being almost impossible to achieve.
Now, Aiichiro Nagaki and Jun-ichi Yoshida, at Kyoto University, and their colleagues have developed a fast micromixing technique that enables highly chemoselective reactions of unstable organolithiums with a variety of electrophiles. Central to their new technique is a flow microreactor with a number of separate microtube reactors and micromixers. They can introduce different reactants through each reactor, which are then mixed in the micromixers. Controlling the flow rates and reagent mixing allows the researchers to take advantage of the short residence times of reaction intermediates to obtain the desired products.
The technique is based on flash chemistry, where microreactors are used to perform fast and highly controlled organic reactions. The team demonstrated it on a wide range of organic substrates to produce a variety of poly-functionalised compounds.
Thomas Wirth, an expert in microreactor technology for dangerous reactions at Cardiff University, UK, praises the research: ‘Taming such unstable and highly reactive species is a further demonstration of the power of flow synthesis and highlights the potential to introduce novel protecting group-free pathways in synthesis planning.’ And Sheryl Wiskur, a synthetic organic chemist at the University of South Carolina, US, says the technique ‘highlights the challenges that can be overcome through the blending of chemistry and engineering’. However, she warns that the technique is ‘really only possible though the use of a microfluidic system’, and notes that addressing this issue could allow for higher yields.
References
This article is free to access
A Nagaki et al, React. Chem. Eng., 2017, DOI: 10.1039/c7re00142h










No comments yet