Japanese chemists are looking to direct cross-coupling of C–H bonds to build graphene from the bottom up
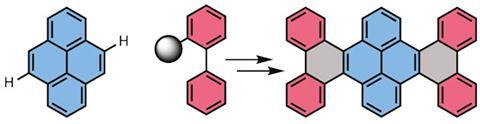
Japanese scientists are making tiny fragments of graphene using direct cross-coupling of C-H bonds to determine what effect size and edge geometry have on the properties of carbon materials. By bolting together aromatic hydrocarbons, they can build nanographene fragments with defined shapes in an attempt to relate geometry to performance.
Speaking at the RSC’s seventh International Symposium on Advancing the Chemical Sciences in Edinburgh, UK, Kenichiro Itami from Nagoya University explained that there is currently no efficient way of making graphene with well-defined shapes and edge geometries from the bottom up. Fabrication technology that made this possible would be exceptionally useful as evidence from graphene device researchers points to the edges being extremely important in determining the properties of a piece of graphene.
In an effort to tackle this problem, the team took the polycyclic aromatic hydrocarbon pyrene and bolted on a biphenyl group on either side of the molecule by selectively activating two of the pyrene’s peripheral C–H bonds and reacting them with a biphenyl boroxine coupling partner, followed by an oxidative coupling to activate two more pairs of C–H bonds and connect up the ring systems into a flat nanographene sheet.
Itami says that while some methods using traditional Suzuki cross-coupling reactions have already been developed, his team’s method has the advantage of not needing to prefunctionalise the hydrocarbon substrates. ‘There are many different polycyclic aromatics you could start from that are commercially available,’ he says, so the method can easily be modified to make all sorts of different nanographenes.
‘This is an excellent example of the potential of C–H functionalisation to address important challenges in materials chemistry,’ says Igor Larrosa from Queen Mary, University of London, UK. ‘Bottom up synthesis of graphene is extremely exciting and will lead to a better understanding of its properties and perhaps to the development of new materials.’
One of the questions Itami is looking to answer is how many rings are needed for nanographene to start behaving like bulk graphene. ‘We just don’t know,’ he says. ‘If I had to guess I’d say it could be around 50–100 rings, but it’s very hard to say – that’s why this work is so interesting.’
Itami also hints that his team is exploring ways to make nanographene that incorporate five or seven-membered rings, such as those found in fullerenes, to explore non-planar topologies of carbon materials, which could show unusual physical and electronic properties.
















No comments yet