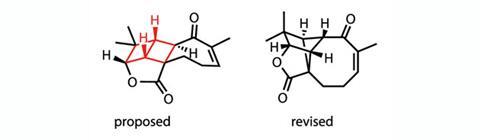
Aquatolide attracted the attention of Dean Tantillo and his team at the University of California, Davis (UCD), because of its unusual ladderane structure – the polycyclic core of the molecule was reported to contain two four-membered rings of carbon atoms fused along one of their C–C bonds, resembling a ladder. ‘As a group, we’re interested in both terpenes and ladderane natural products,’ says Tantillo. ‘At the time we came across aquatolide, it was one of only two ladderane natural products I knew of that wasn’t a lipid.’
Having dabbled in NMR prediction before, Tantillo decided to model the spectra to check whether the structure was right before investing time and effort in trying to work out how it might be made naturally. ‘When we did that, the structure was clearly not correct,’ he says.
Jonathan Goodman from the University of Cambridge, UK, has developed tools for distinguishing related structures by matching multiple sets of experimental and/or predicted data. ‘But what Tantillo’s group is doing is putting less information in – they’ve got one structure and one spectrum, and are trying to decide whether it’s right or not,’ he says. This only really works well if there are significant differences between the predicted and experimental spectra, as there were for aquatolide. ‘Often you can do the calculations and it looks different, but not that different,’ Goodman adds. This won’t lead to a firm conclusion, but can be enough to prompt further investigation.
Unfortunately, as Tantillo explains, turning the calculations around to go from a spectrum to a structure is much more difficult. ‘We looked at a couple of hundred other possibilities and calculated the spectra for those and they were also clearly wrong,’ he says. Eventually, they hit on a structure that they were reasonably confident was correct, but it had a significantly different skeletal structure to that previously proposed.
It was then that Jared Shaw, one of Tantillo’s colleagues at UCD, mentioned that he might be able to get hold of some samples of Asteriscus aquaticus, a small plant related to the daisy, from which aquatolide had been isolated. ‘So we decided to try to re-isolate it and do some additional experiments,’ Tantillo says. ‘It was a good exercise, because Jared is a natural products chemist with a healthy scepticism of calculations, so I figured that if we could convince him, we could convince anyone.’ Extensive NMR analysis of the isolated aquatolide matched the predicted structure very well and, eventually, x-ray diffraction confirmed the structure conclusively.
Tantillo is quick to stress that this is not the first time natural product structures have been revised using NMR calculations. However, he believes the strength of the theory is now such that, in many cases, it could be routinely used by synthetic or natural products chemists to check the credibility of their structures. Goodman agrees, saying that this is a great example of calculation and experiment working together. ‘When they work together, you can get to a definitive conclusion much more quickly than with either by themselves,’ he says.







No comments yet