A reaction common to synthetic organic chemistry has been fully integrated into a microbe, expanding the scope of organic products that could be biosynthesised. The proof-of-concept work, which incorporated carbene-transfer reactions into the metabolism of a strain of Streptomyces bacterium, demonstrates the possibility of a scalable microbial platform for biosynthesising natural and new-to-nature products.
Biosynthesis offers an environmentally friendly and sustainable way to make many natural, as well as new-to-nature products. But many reactions that are available to organic chemists – including carbene-transfer reactions – are not naturally performed by organisms.
Carbene-transfer reactions involve a precursor, such as an azide or diazo compound, that loses nitrogen atoms to generate a carbene – a divalent carbon atom with two substituents. The carbene can then reacts with a chemical, such as an alkene, to effectively transfer a carbon to make a new molecule such as cyclopropanes, which are increasingly incorporated into medicinally active compounds.
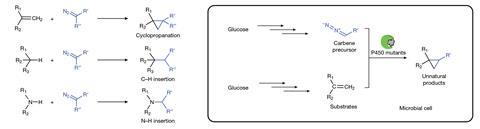
In recent years, researchers have shown that carbene transfers, catalysed by engineered metalloenzymes, can be performed in cells and used for biosynthesis. But with no way for the organism to make the carbene and alkene reagents, these had to be added to the cell, which is too expensive to scale up and limits the substrates that can be used. Although an artificial biosynthetic pathway for generating an alkene component in E. coli had been achieved by John Hartwig’s lab at the University of California, Berkeley and collaborators, the catalyst and carbene precursor still had to be added.
Now, Hartwig, together with Jay Keasling’s group at the University of California, Berkeley, Aindrila Mukhopadhyay at Lawrence Berkeley National Laboratory, and their colleagues have fully integrated carbene-transfer chemistry into the metabolism of Streptomyces albus, which generates every component required – carbene precursor, alkene and catalyst – for the entire synthetic pathway from glucose to unnatural cyclopropanes.
‘Incorporating organometallic chemistry into biosynthesis has been a long-time dream of mine, and there it was,’ says Hartwig. ‘Of course, much needs to be done to make such biosynthesis practical, but we all made it happen, led by a superb postdoctoral researcher and graduate student Jing Huang and Andrew Quest, and bolstered by the many collaborators.’
Microbial mutant
First, the researchers set out to identify a natural product that could act as a biocompatible carbene precursor. In vitro experiments identified the amino acid azaserine, which was shown to undergo carbene transfer to cyclopropanate the alkene styrene. The team then searched for the biosynthetic pathway of azaserine by delving into the genome sequences of two microbes that are known to naturally make it. The gene cluster thought to be responsible was then cloned and integrated into S. albus.
With S. albus as a suitable host and able to make the carbene precursor, the team turned to finding a catalyst. Knowing that several cytochrome P450 enzymes and their mutants had previously been shown to catalyse cyclopropanation by carbene transfer, the researchers used E. coli to express the enzymes’ encoding genes to screen them for activity towards the reaction of azaserine with styrene. The team arrived at a cytochrome P450 naturally found in thermal spring-dwelling microbes. This had the highest activity and it was then engineered with five rounds of evolution to boost its performance, producing moderate yields of cyclopropanes.
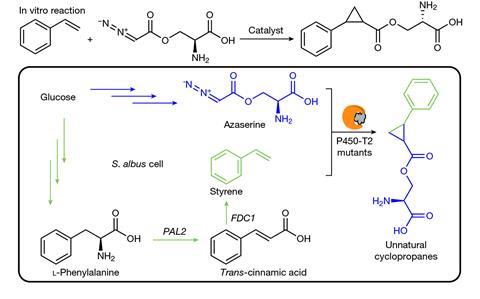
Lastly, the alkene component of the reaction, styrene, was then incorporated into S. albus. The biosynthetic pathway of styrene had previously been established in E. coli and a species of fungus but not in Streptomyces. To do this, the researchers integrated the required genes for the pathway into the genome of the azaserine-producing S. albus strain. Altogether, the final engineered S. albus was shown to biosynthesise all of the reaction components for a carbene-transfer reaction and produce unnatural cyclopropanes.
‘This a very elegant piece of work,’ comments Paul Race who investigates biosynthesis at the University of Bristol, UK. ‘The establishment of a biosynthetic route to the diazo ester carbene precursor is an impressive outcome. However, the subsequent marriage of this with an engineered P450 to cyclopropanate an intracellular target compound is a major step forward, effectively unlocking a “non-natural” metabolic process.’
Rudi Fasan who studies chemo-biosynthetic strategies at the University of Rochester, US, is equally impressed. ‘The possibility of assembling biosynthetic pathways for the production of diazo-containing metabolites in engineered microbial strains is an exciting advance,’ he says. ‘As new examples of these rare but very interesting natural products and biosynthetic pathways are discovered in nature, one can envision important applications of this approach toward expanding the range of interesting molecules obtainable by biomanufacturing with custom-made microorganisms.’
References
J Huang, Nature, 2023, DOI: 10.1038/s41586-023-06027-2









No comments yet