For the first time, researchers in Japan have installed multiple aryl groups onto a cubane framework. Cubane’s (C8H8) eight sp3-hybridised carbon atoms make it a rigid and unique molecule. Its derivatives are highly sought-after, but often limited to substitution at the 1- and 4-positions thanks to the commercially-available 1,4-cubanedicarboxylic acid precursor. Late-stage C–H transformations of cubane have been explored before, but it has only previously been possible to install halogen, carboxylic acid, phenyl and hydroxyl groups.
Starting from 1,4-diamidocubane, the team, led by Akiko Yagi and Kenichiro Itami of Nagoya University, first synthesised the monoarylated cubane by directed ortho C–H metalation followed by palladium-catalysed arylation. When they repeated these two steps with the product, however, the diarylcubane did not materialise. But by changing one of the amido groups to a cyano group, the acidity of the cubane was enhanced – and this meant it was possible for them to make the diarylcubane by directed ortho C–H metalation/arylation.
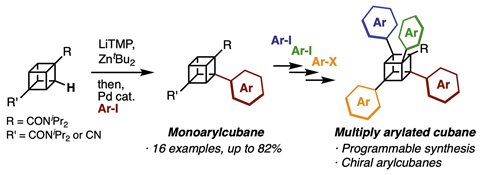
This second arylation was regioselective, and took place at the C–H bonds near the amido group. They proceeded to synthesise the tri- and tetra-arylcubanes from the diarylcubane, using similar reaction steps to before.
The programmable, predictable method enabled the synthesis of a wide range of mono-, di-, tri- and tetra-arylated cubanes. Such families of chiral cubane derivatives give access to fresh areas of chemical space, and have the potential to feature in pharmaceuticals and functional materials.







No comments yet