Theoretical scientists have predicted the first stable hexavalent planar gallium structure.1 The simulated cluster maintains a flat structure due to double aromaticity and a balance of steric forces, and isn’t stable when other group 13 elements replace gallium.
Under the right circumstances it’s possible to not only coax more bonds out of an atom than its number of valence electrons – hypervalency – but to constrain these bonds in a plane. For the main group elements clusters with these structures are scarce, even in simulations: some planar hexacoordinate carbon centres have been devised,2 and boron3 and its group 13 peer aluminium are also predicted to form planar hexacoordinate centres.
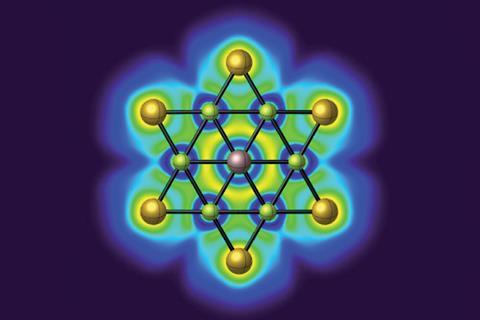
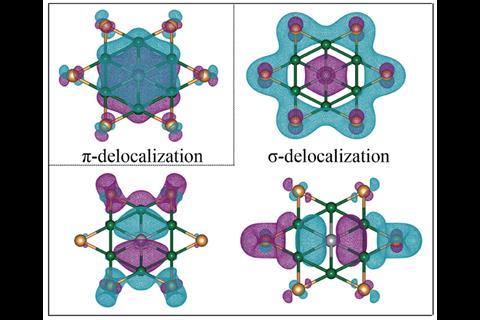
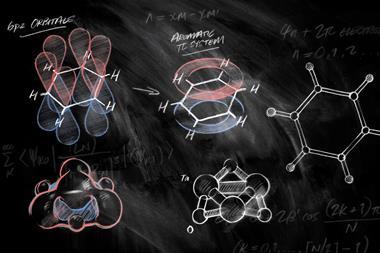
In a new study, Sudip Pan of Philipps-University Marburg in Germany, Zhong-hua Cui of Jilin University in China, and their colleagues have devised the first cluster with a stable hexacoordinate planar gallium centre, GaBe6Au6+, using a central gallium atom surrounded by concentric rings of beryllium and gold. The planar ring system isn’t stable on its own, but interacting with gallium sets up a doubly-aromatic structure of σ and π orbitals delocalised across the whole cluster. Like a recently-devised planar hexacoordinate boron cluster,3 the π orbital is occupied by an electron each from the gallium and the ring system, while the σ orbitals represent dative bonds to and from the central atom.
For smaller, less electronegative aluminium these bonding interactions are weaker and the structure is unstable. With the larger indium and thallium, the planar system is destabilised by steric repulsion, although with thallium the planar structure is a high-energy local minimum.
References
1 S Pan et al, Chem. Sci., 2021, DOI: 10.1039/d1sc05089c
2 L Leyva-Parra et al, Angew. Chem., Int. Ed., 2020, 60, 8700 (DOI: 10.1002/anie.202100940)
3 A J Kalita et al, Chem. Commun., 2020, 56, 12597 (DOI: 10.1039/d0cc05668e)








No comments yet