A far more efficient and selective catalyst for electrochemically reducing carbon monoxide to acetate has been developed by an international collaboration headed by researchers in China and Canada. They found that less is more when it comes to the copper catalyst, and that reducing the amount used actually inhibits side reactions, producing more acetate.
More than 18 million tonnes – worth nearly £8 billion – of acetic acid are produced every year as solvents and precursors to other chemicals used in paints, adhesives, food additives and other applications. The standard synthesis starts with syngas and generates 1.6kg of carbon dioxide-equivalent emissions per kilogram of acetic acid. Acetate can instead be produced electrochemically from carbon dioxide or carbon monoxide using copper-based catalysts. ‘If you look at the metals in the periodic table, copper is the only metal that balances the adsorption strengths of the intermediates that we care about to facilitate carbon-carbon coupling,’ explains Joshua Wicks at the University of Toronto in Canada. The acetate is just one chemical in a mixture of ethylene, ethanol, 1-propanol and others.
Several groups have already shown that the mechanism containing the ‘monodentate’ intermediate, which is bound to the surface at only one point and protrudes perpendicularly outwards, proceeds on to acetate, whereas the bidentate intermediate produces other unwanted compounds. Instead of covering a significant proportion of the catalyst surface with copper, therefore, in the new work Wicks and colleagues led by the University of Toronto’s Ted Sargent and Yuanjie Pang at Huazhong University of Science and Technology in China used a silver surface doped with only 1% copper. ‘If the surface is all copper and the carbon in your carbon–carbon–oxygen adsorbate likes to adsorb on copper, then you can have a mixture [of monodendate and bidentate intermediates],’ explains Wicks. ‘But if we…keep constraining the size of the domains, we begin to constrain the adsorbates into monodentate orientations.’
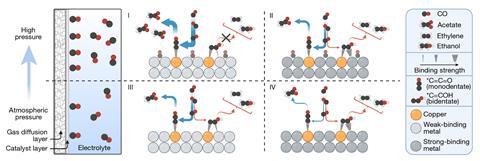
The researchers set up an aqueous electrochemical cell with a gaseous inlet stream. When the partial pressure of carbon monoxide was 101kPa, acetate selectivity was 69%. This was about 1.4 times the previous record, but was restricted by the proportion of electrons being diverted to simply electrolysing the water because of the dispersed nature of the copper domains active for carbon monoxide reduction. The researchers therefore increased the amount of carbon monoxide to compensate: when the CO partial pressure was 1MPa, the selectivity for acetate was around 91%.
The researchers do not anticipate that the need for a carbon monoxide feed will pose significant difficulties. ‘The CO2 to CO electrochemical reaction in solid oxide electrolysis cells and other room temperature electrolysis cells has been well documented, and there are companies running these reactions at pilot scale and beyond,’ says Wicks. ‘There is never any intention to source fossil-fuel CO.’
‘This opens a unique direction,’ says Haotian Wang of Rice University in Texas. ‘The performance is excellent, the stability is very good, the selectivity is excellent. We still have a lot of limitations in our preparation tools and our methods to understand the reaction mechanism, but I don’t think that should be called a shortcoming so much as a future research direction… We should further expand this strategy to other catalytic materials systems.’
















No comments yet