Scientists in the US have used subtle electrochemical effects to isolate and separate tiny plastic particles in suspension.
The method, devised by Collin Davies and Richard Crooks at the University of Texas at Austin, involves piping a Tris buffer solution containing the microbeads through a forked microscopic channel, in the presence of an electric field.
Being negatively charged, the particles’ direction of travel depends on a battle of two opposing forces: the pull of the electric field, and the push of the moving fluid. If the field varies across space, then so does the victor in this battle, shepherding the particles to a sweet spot where the forces are matched.
Davies and Crooks achieved such a field variation by placing conducting wires along the channel – a technique called faradaic ion concentration polarisation (fICP). Electron flow induced in each wire turns its ends into an impromptu pair of electrodes, driving an electrochemical reaction in the solution. This in turn alters the local ion concentration, which disturbs the fluid’s conductivity, and voilà – a gradient in the electric field results.
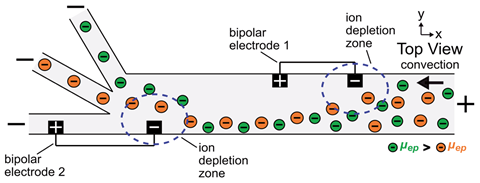
But different plastics feel different strengths of pull in an electric field, meaning they settle at separate sweet spots. By exploiting this distinction, the researchers could marshal two microplastics down different branches of their forked junction, thus separating the mixture.
Microplastics are a widespread pollutant with grave effects on health and the environment. Removing, retaining and recycling these damaging waste fragments is an urgent challenge. Davies and Crooks aim to adapt their method to work without a buffer solution, making it a more versatile means of sorting microplastics.
References
This article is open access
C D Daviesa and R M Crooks, Chem. Sci., 2020, DOI: 10.1039/d0sc01931c




















No comments yet