Chemists can now precisely control the stereochemical sequence of synthetic polymers using similar techniques to those used to create artificial DNA. Growing polymers one monomer at a time could unlock ‘out of the box’ sequences with unique properties, say researchers.
Synthetic polymers are typically non-uniform, varying in both length and structure. Current synthetic methods – such as radical or anionic polymerisation – also have limited control on how stereocentres are introduced along the polymer backbone. ‘I was a bit frustrated by the tools [chemists] use to build polymers,’ says Jean-François Lutz at the University of Strasbourg in France who led the study.
Lutz and Ranajit Barman have now developed a synthetic method that can create polymers with complete control over the order of monomers, allowing for the relative order of stereocentres to be precisely managed.
The team first synthesised two chiral phosphodiester amide monomers with opposite configuration, which were then used in iterations of solid phase phosphoramidite chemistry to build the polymers stepwise, similar to how artificial DNA and RNA is made.
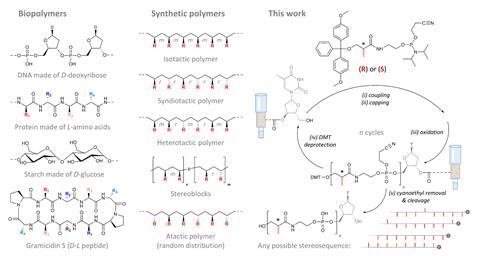
Twenty different polymers up to 50 monomers long were synthesised using this method, including polymer sequences that were previously difficult to obtain. ‘The polymers that we made are not super interesting [in terms of their properties],’ says Lutz, adding that they were made as a proof of concept that the method worked.
There are currently only three main types of stereochemical polymers: isotactic, meaning all substituents are on the same side of the chain; syndiotactic, an alternating substituent configuration; and heterotactic, where the stereochemistry changes every two monomers, explains Lutz. ‘These terms are historical,’ he says, adding that this new method unlocks the possibility for ‘out of the box’ sequences that have not yet been made. With this method, over one million unique sequences are theoretically possible for polymers with as little as 20 monomers that contain stereocentres.
‘The huge disadvantage of this solid phase synthesis method is that it is low scale due to cost,’ says polymer chemist Róża Szweda at Adam Mickiewicz University in Poland. She thinks that this may hinder the application of these polymers for material use. Szweda suggests that these polymers may find alternative uses in encoding digital information or helping create artificial enzymes, which only need small amounts of polymer.
In addition to the spectroscopic and mass spectrometry techniques used by the team, Szweda says that advanced 2D-NMR may help further confirm the stereochemistry of these polymers. She adds that this method may subsequently ‘open up a demand for new characterisation techniques’ that can probe chiral sequences more accurately.
Lutz says that he now hopes to use the same strategy to synthesise polymers with different monomers. ‘We are working on making polymers where the stereocentres are closer to each other … as there is much more influence on [polymer] properties with this neighbouring effect,’ Lutz says.
References
R Barman and J-F Lutz, J. Am. Chem. Soc, 2025, DOI: 10.1021/jacs.5c15603














No comments yet