A polymer can carry insulin through the skin and into the bloodstream, heralding a future where people with diabetes could manage their condition without injections. The approach, which has shown promise in animal and human cell models, might one day enable transdermal delivery of other biological drugs.
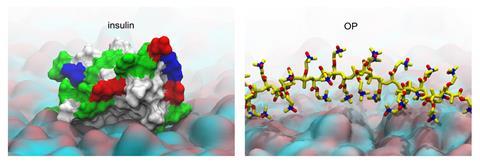
Skin is an excellent barrier, making it difficult for large molecules, such as insulin, to pass through. The outermost layer of the skin is coated in fatty acids that make it hydrophobic, while the layers beneath it are hydrophilic. Only small, oily molecules can pass through the top layer. Larger, water-soluble molecules, including virtually all peptides and proteins, cannot penetrate this surface. As a result, protein-based drugs like insulin still need to be injected. ‘There are many attempts to break this top barrier,’ explains Youqing Shen, a polymer chemist at Zhejiang University, China, who led the work.
We use this polymer as a locomotive and insulin as a cargo
Youqing Shen, Zhejiang University
Several alternative delivery routes have been explored, but each comes with drawbacks. Microneedle patches puncture the skin directly, while high-pressure jet injectors, electrical devices and ultrasound approaches push insulin through the skin by briefly disrupting the barrier. However, these methods compromise skin integrity, causing discomfort and increasing the chances of infection, reducing patient compliance. ‘These approaches have been tested in labs, but are not applicable for frequent use because they damage the skin or change its structure, causing side effects,’ says Shen. Oral administration of insulin has also been investigated. ‘But insulin would be digested in the stomach so this doesn’t work either,’ notes Shen.
To tackle this challenge, the researchers developed a zwitterionic polymer (OP) – both positively and negatively charged – which uses a pH-dependent charge switch to traverse the skin barrier. The skin’s surface is slightly acidic, so when OP is applied to it its amine groups become protonated, giving the polymer a positive charge. This allows it to interact with the fatty acids and lipids of the top layers of the skin and diffuse through it, providing an entry point where most biomolecules fail.
As OP moves deeper into the skin where the pH is neutral, it transforms into a zwitterionic form. ‘Here, OP loses the cationic charge and becomes electrostatically neutral,’ says Shen. In this state, it no longer sticks to cell membranes and instead slips between them. Imaging experiments showed OP travelling through the major skin layers, before entering small vessels that drain into the bloodstream.
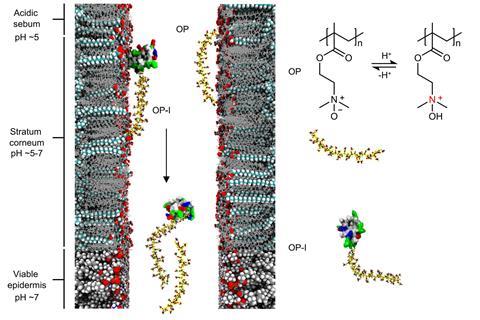
Using click-chemistry techniques, the team then attached human insulin to the polymer. ‘We use this polymer as a locomotive and insulin as a cargo,’ explains Shen. Fluorescent tracking confirmed that OP–insulin crossed the skin in both mice and minipigs, whereas free insulin remained on the surface. ‘We found that insulin conjugated with this polymer has almost the same activity as the original,’ says Shen.
‘The work stands out not just for its pharmacological effect but for the mechanistic intent behind the carrier design,’ comments Nazila Kamaly, an expert in synthetic biomaterials and drug delivery at Imperial College London, UK. ‘There is a clear design principle – pH-programmed, zwitterionic carriers with defined membrane affinity as a viable route for transdermal biologic delivery.’
In tests with type 1 diabetic mice, a topical dose of OP–insulin lowered blood glucose to healthy levels within an hour and kept it controlled for as long as 12 hours – significantly longer than injected insulin in the same model. In diabetic minipigs, whose skin is more similar to humans, a lower dose was enough to normalise blood glucose without causing irritation or barrier damage.
‘The combination of mouse, minipig and 3D reconstructed human skin provides a reasonable preclinical foundation,’ says Kamaly. ‘The work is promising, but human trials are now needed.’
References
Q Wei et al, Nature, 2025, DOI: 10.1038/s41586-025-09729














No comments yet