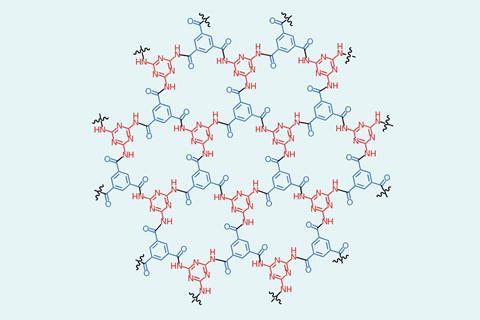
Polymers formed from two-dimensional analogues of covalent organic frameworks (COFs) can be almost as impermeable to gases as graphene, researchers in the US have found.1 The work could open up a wide range of applications, from protecting perishable substances against oxidation to resonators in mobile phones.
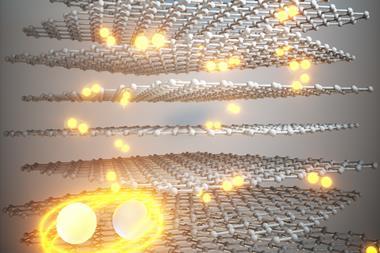
COFs are similar to the metal–organic frameworks (MOFs) that were awarded the 2025 Nobel prize in chemistry but, whereas in MOFs the organic linkers are connected by metal ions, in COFs the central nodes are themselves organic groups. COFs usually form cage-like structures with a huge internal surface area that can absorb gases such as carbon dioxide. In 2022, however, chemical engineer Michael Strano at the Massachusetts Institute of Technology and colleagues showed that spin casting a 2D polyaramid on a silica-covered silicon wafer could also produce ultrastrong hydrogen-bonded polymer networks.2 ‘We polymerised in two dimensions. Instead of making one-dimensional noodles … we were able to make two-dimensional platelets,’ says Strano. Ironically, the networks that resulted had almost no free volume.
The new study was inspired by observations in the 2022 paper. They had deposited the material on a wafer containing small square wells to allow them to create bridges that could be probed with a nano-indenter to measure the material’s mechanical strength. ‘It’s common when you spin coat a film over little wells, you can trap gas and you’ll make these bubbles,’ Strano says. ‘But we noticed that we still had these bubbles that we made in 2021.’
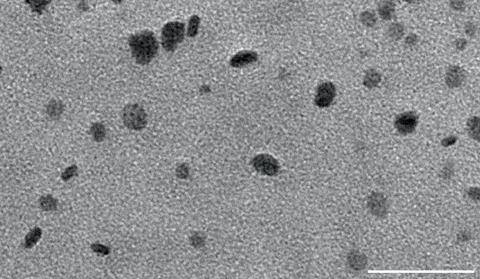
The researchers created new films of the material between 4nm and 65nm thick and investigated their permeability to various gases. They found that a 35nm-thick layer could even trap molecules as small as nitrogen for over three months. The researchers’ models suggest this is because the 2D layers stack with random relative orientations, so a molecule has difficulty passing through multiple layers. ‘By the time you’re on maybe the fourth or fifth layer, [the material is] impermeable,’ says Strano.
The only other thin material with this kind of impermeability is pristine graphene, which has to be exfoliated, whereas the team’s polymer can be processed like any other polymer. The researchers demonstrated that a 60nm-thick layer of the material could extend the lifetime of a methylammonium lead iodide perovskite solar cell sevenfold by excluding oxygen and water.
Strano says that the material could be used to increase the shelf life of perishable foods and medicines and reduce the amount of plastic needed to do so. The material’s strength and chemical inertness was also used to produce a polymer nano-electromechanical resonator. Resonators, which are required for frequency selectivity in devices like mobile phones, are currently produced from specially-grown crystalline materials such as lead zirconate titanate. Replacement with a polymer could potentially make the devices much cheaper and more energy efficient.
‘It must be, per unit thickness, the highest barrier [to gas penetration] that’s been created for a single, standalone polymer,’ says polymer chemist Jaime Grunlan at Texas A&M University in the US. ‘If it is true then that’s a significant thing.’ He cautions, however, that ‘the most important property of any material is cost’, and says that a material just synthesised in the laboratory is unlikely to be a viable food packaging material on these grounds alone. ‘The next steps would not be sexy,’ he says, but would instead involve studying if and how large-scale commercial production is economically viable.
References
1 C L Ritt et al, Nature, 2025, 647, 383 (DOI: 10.1038/s41586-025-09674-9)
2 Y Zeng et al, Nature, 2022, 602, 91 (DOI: 10.1038/s41586-021-04296-3)












No comments yet