Chemists make stable compound featuring a silicon atom that forgoes tetrahedral geometry
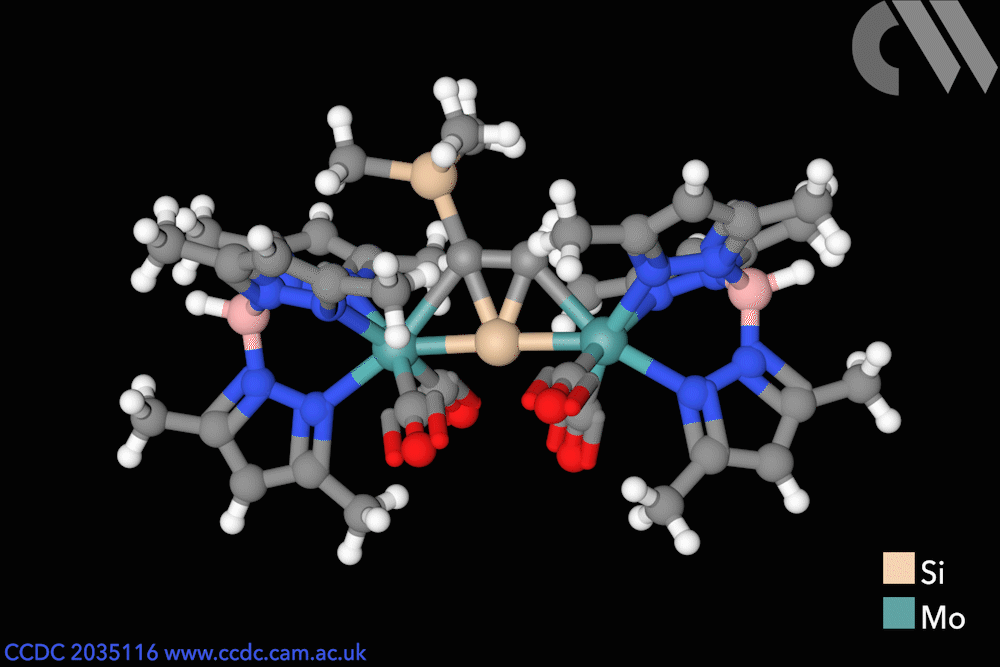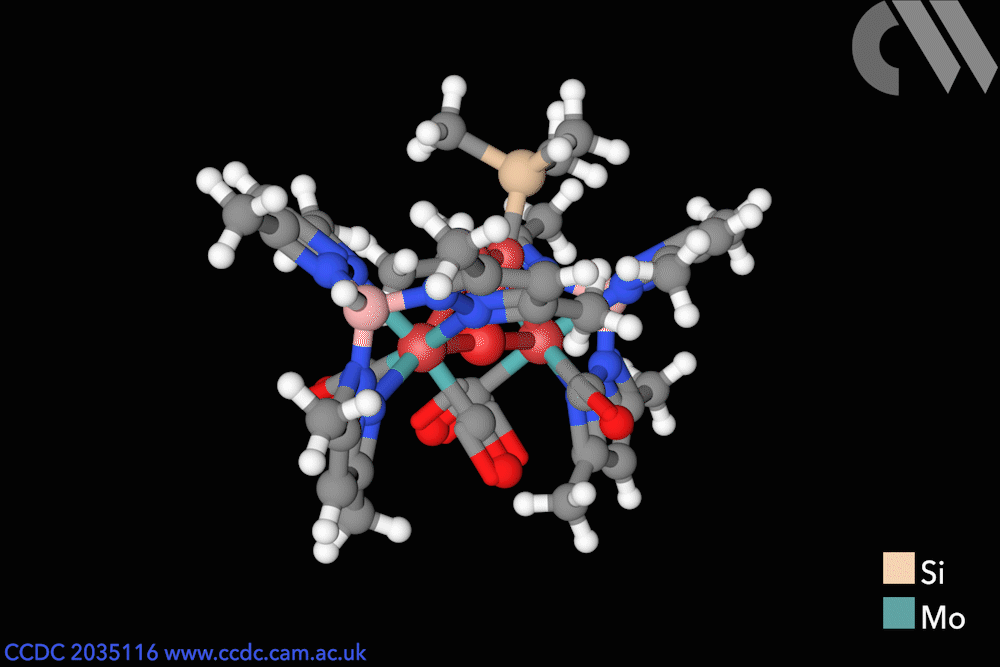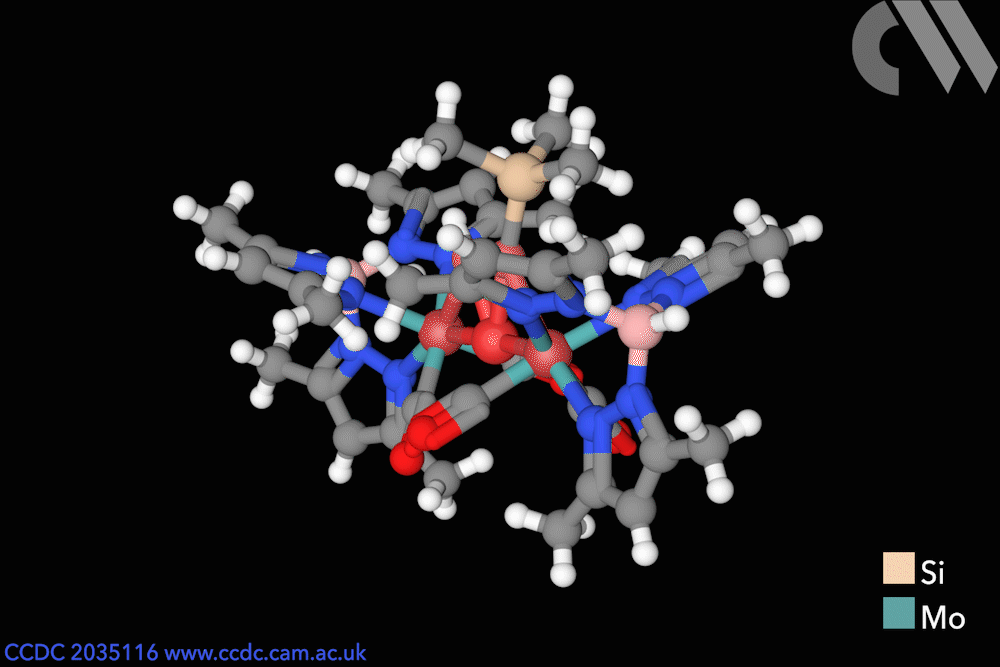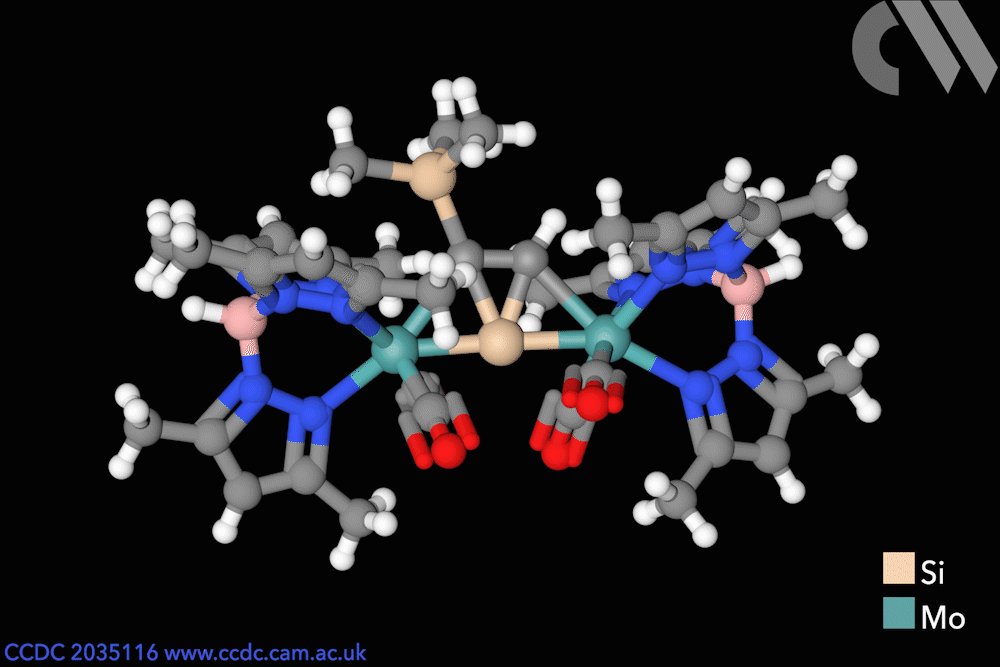
Silicon has entered flatland with the first stable compound featuring a planar – rather than tetrahedral – tetracoordinated silicon atom. The unusual molecule was made possible by stabilisation coming from two metal centres as well as an aromatic core.
In 1874, chemists Joseph Le Bel and Jacobus van ‘t Hoff recognised independently that carbon prefers a 3D, tetrahedral arrangement for its substituents. Indeed, flattening a 3D molecule like methane would require an enormous amount of energy – more than 600kJ per mol. But that didn’t stop researchers from trying to make planar tetracoordinated carbon compounds, the first of which was reported in the late 1980s.
But flat tetravalent silicon has proven harder to make, despite the fact that deforming the silicon analogue of methane – tetrahydrosilane – from tetrahedral to planar has been calculated to require significantly less energy (450kJ per mol). Only one example of flat silicon was known, a gas-phase species that had never been isolated.
Chemists in Germany have now created the first thermally stable silicon compounds violating the van ‘t Hoff–Le Bel concept. The planar silicon is part of a tricyclic core featuring three fused molecular triangles, one of them containing two flat carbon atoms alongside the flat silicon.
The bright yellow compounds are only moderately air sensitive and don’t decompose until heated to more than 200°C. Weak bonds between the carbon atoms and the two metal atoms on either side – either molybdenum or tungsten – help stabilise the structure. Moreover, the central silicon–carbon triangle receives extra stabilisation from being aromatic, as it has two delocalised π electrons.
References
P Ghana et al, J. Am. Chem. Soc., 2020, DOI: 10.1021/jacs.0c11628
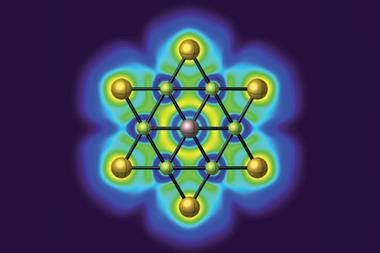
![An image showing a [M2(BzN6-Mes)]n− complex](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/4/0/8/514408_index_128835.jpg)






No comments yet