A theoretical investigation has identified the first planar tetracoordinate fluorine-containing systems: FIn4+, FTl4+, FGaIn3+, FIn2Tl2+, FIn3Tl+ and FInTl3+.1 While other planar tetracoordinate systems with carbon, nitrogen and oxygen are known, until now fluorine equivalents had not been reported.
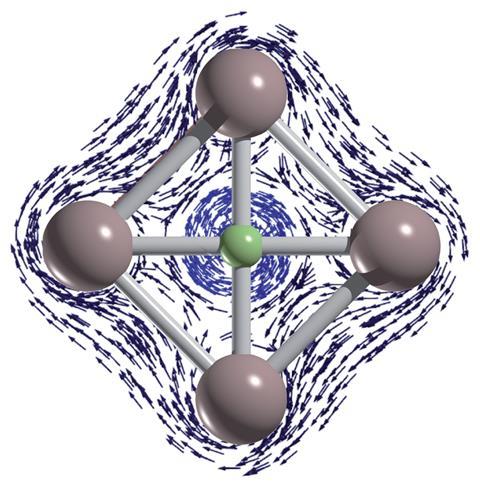
The researchers, led by Jorge Barroso and Gabriel Merino from Cinvestav in Mexico, explored the potential energy surfaces of viable pentaatomic clusters with a fluorine atom combined with main group elements, which had 18 valence electrons and charges from -2 to +2. This led to 440 combinations and around 100,000 optimisations.
They only found planar tetracoordinate fluorine systems with substituents from group 13 elements – gallium, indium and thallium. The cavity formed in the centre of four aluminium atoms was not large enough to accommodate a fluorine atom. The size of this cavity is very important, and both indium and thallium formed clusters with sufficiently large cavities, giving FIn4+ and FTl4+.
Replacing a gallium atom in FGa4+ with the heavier thallium was unsuccessful: the F–Ga bond lengths became shorter, so the cavity was too small for a fluorine atom. They discovered the other four clusters by substituting in different elements, which gave cavities that could accommodate a fluorine atom.
Planar tetracoordinate fluorine atoms proved challenging to identify, and the researchers realised why when they compared CAl42- and FAl4+. Carbon acts as a σ-acceptor but compensates by back-donating its 2pz electrons into the π-bonding. This back-donation does not occur with fluorine, with consequences on the induced magnetic field and aromaticity.
If chemists can go on to make these fluorine clusters, it will join other recent research exploring unusual fluorine species.2
References
These articles are open access
1 G Castillo-Toraya et al, Chem. Sci., 2021, DOI: 10.1039/d1sc01325d
2 P Pröhm et al, Chem. Commun., 2021, DOI: 10.1039/d1cc01088c
![[Br4F21]- index image](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/1/7/3/532173_br4f21indeximage_49094.jpg)
![An image showing a [M2(BzN6-Mes)]n− complex](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/4/0/8/514408_index_128835.jpg)







No comments yet