Complex with one of the shortest uranium–transition metal bonds ever reported is unstable in solution
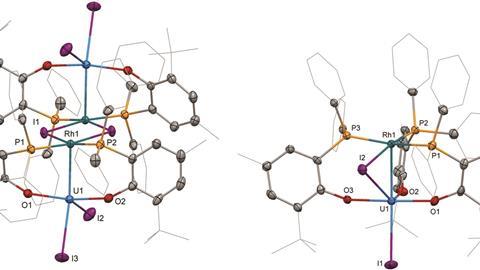
Researchers in the UK have made the first two uranium–rhodium complexes and found their uranium–rhodium bonds to be among the shortest heterometallic uranium bonds ever reported.1
Building on their recent success forming uranium complexes with nickel, palladium and platinum,2 Polly Arnold’s group at the University of Edinburgh used a carefully designed bidentate phosphinoaryloxide ligand (ArPO–) to create two distinct uranium–rhodium complexes: a tetrametallic dimer, [I2U(OArP)2RhI]2, and a monomeric complex with three phosphinoaryloxide ligands and a bridging iodide. Although the two uranium–rhodium bonds are of similar length (2.760Å in the dimeric complex and 2.763Å in the monomeric one), electrochemical studies show that the bond stabilities are very different.
For the dimeric complex, electrochemical studies show that the reduction is localised on the uranium centres, implying that there is no electronic communication between uranium and rhodium. In fact, the complex dissociates in solution. The monomeric complex, however, does show evidence of a weak interaction and is stable in solution.
Arnold proposes that the three phosphinoaryloxides in the monomeric complex force the filled rhodium dz2-orbital towards the uranium centre – as seen in the previously reported complexes2 – to create a weak interaction between the metals. A frontier orbital delocalised across the two metals through the bridging iodide – not seen in the dimer – then stabilises the longer uranium–rhodium bond, showing us that shorter metal–metal distances do not necessarily result in strong metal–metal bonds.
References
1. J A Hlina et al, Dalton Trans., 2017, DOI: 10.1039/c6dt04570g (This article is free to access until 30 March 2017.)
2. J A Hlina et al, J. Am. Chem. Soc., 2016, 138, 3333 (DOI: 10.1021/jacs.5b10698)





![Structure of [UCl4(HCN)4]](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/4/5/2/135452_The-structure-of-the--UCl4-HCN-4--molecule-in-its-solid-state-compound.jpg)







No comments yet