
Extremely light, floating films can make foul water fit to drink again by using light to kill harmful bacteria. The film, comprising a photocatalyst that generates long-lasting bactericidal radicals, is quite potent under low light intensities and can purify water even under smartphone light.
Lack of access to safe drinking water claims up to 2 million lives annually, including a child under five every two minutes. This crisis is compounded by the fact that 81% of the at–risk population lives in low- and middle-income countries and remote villages that are plagued by poor infrastructure that often have no centralised water treatment plants.
These vulnerable communities have to rely upon point-of-use disinfection systems but they too suffer from certain limitations: UV irradiation is energy intensive, chlorination produces carcinogens, filtration fails to disinfect completely and solar disinfection needs impractically long periods of time, especially under cloudy conditions.
Photocatalytic systems look promising but they need high-intensity light (≥100mW/cm2) to be of any practical use, a requirement which even the brightest places on Earth – on average receiving 33–35mW/cm2 of sunlight – cannot supply. Also, for their bactericidal action, they mostly rely on reactive oxygen species, which can be so short-lived that they don’t kill all bacteria, and so reactive that they end up corroding the photocatalyst and adding novel contaminants to the water.
To circumvent both these shortcomings of conventional photocatalytic systems, researchers at Sun Yat-sen University, China synthesised a photocatalyst by coupling a carbazole carrying an electron-donating phenylalkoxy chain with anthraquinone, which produces a long-lived active species called oxygen-centred organic radicals (OCORs) on excitation by light. They incorporated this photocatalyst into a fibrous photocatalytic film made of polystyrene and polyacrylonitrile.
When highly contaminated water covered with the film was exposed to low light intensity (10mW/cm2), the photocatalyst killed 99% of Escherichia coli within 20 minutes and eradicated all the bacteria in half an hour. In just 40 minutes, a 10-litre sample of water, teeming with up to 20,000 bacterial colonies per millilitre, was rendered safe, meeting the World Health Organization’s safe standards for drinking water. It also killed Staphylococcus aureus within 25 minutes, showing broad spectrum capabilities.
Closer examination of the photocatalyst’s activity revealed that after 30 minutes of light exposure, the OCORs disrupted the bacterial cell membrane, ‘disembowelling’ E. coli and causing its internal contents to spill out, killing them. The OCORs’ killing power was aided by hydrogen peroxide and singlet oxygen.
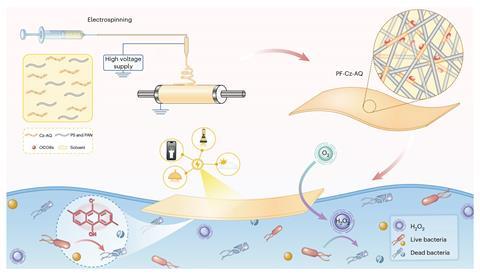
Weighing only 4mg/cm2, this film is portable, floats on water and can be cut to fit the shape of vessels to cover the entire surface of water. It disinfected water even when only illuminated with a smartphone flashlight (5mW/cm2).
‘The standout idea is the use of unusually long‑lived oxygen‑centred organic radicals, which keep working for minutes,’ comments Guihua Yu, a materials scientist from the University of Texas at Austin, US, who wasn’t involved in the work. Though he finds the floating film ‘practically deployable’, he cautions that ‘real‑world rollout will still need more careful checks on cost, long‑term fouling and end‑of‑life handling’.
‘The amount of water treated per mass of material seems impressively high,’ says Michael Wong, a chemical engineer from Rice University, US, who also wasn’t involved in the work. ‘They show high-quality results and strong evidence of real-world usefulness.’ He says the floating film is the ‘coolest part’ of this work as ‘it stands out as an unusual photocatalyst because it is all-polymer (no metal or metal oxide)’, he explains.
References
Y Huang et al, Nat. Water, 2025, 3, 1003 (DOI: 10.1038/s44221-025-00500-0)










No comments yet