A photocatalytic flow process activates simple gaseous alkanes for an atom economic late-stage alkylation of pharmaceutical heterocycles. The inherently scalable process employs cheap and abundant feedstocks to cleanly generate six distinct derivatives and could have a transformative impact on both drug discovery work and manufacturing.
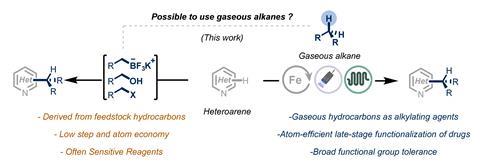
Diversifying lead compounds is an important step to optimise the performance of new drug candidates and, in particular, the addition of simple alkyl side chains can significantly enhance their absorption, distribution, metabolism and excretion properties. However, C1–C4 hydrocarbons themselves are rarely used in this context. The difficulty of working with gaseous reagents, combined with the challenge of selectively activating their strong C–H bonds means existing alkylation methods typically use either toxic or heavily-functionalised precursors and gaseous alkanes are instead burnt as fuels. But, with growing pressure to develop cleaner chemical processes and to phase out fossil fuel energy, methods to repurpose hydrocarbon gases as reagents are being examined.
For Timothy Noël, flow technology provided the perfect solution to this conundrum. His team at the University of Amsterdam in the Netherlands explored photochemical hydrogen atom transfer conditions to activate the stubborn alkane C–H bonds, drawing on the innate benefits of flow reactors to closely control the gas–liquid reaction. ‘Flow and photochemistry pair very well, but it’s also good for gases,’ he says. ‘The gas cannot go anywhere, it cannot escape and it’s very close to the liquid where the catalyst is. So, we found that we could cleave [these] C–H bonds at room temperature.’
The straightforward setup bubbled gas from a detachable canister through a liquid phase containing an iron catalyst and heterocyclic substrate. Under irradiation, the excited catalyst extracted a hydrogen atom from the alkane gas, generating a reactive alkyl radical, which then rapidly attacked the heterocycle in the alpha position, forming only hydrogen as a byproduct.
The four different gases – methane, ethane, propane and butane – generated a total of six products, propane and butane each forming two readily separable isomers. The team then demonstrated the generality of this process across a variety of heterocycles, even enabling late-stage modifications of several natural products and drugs including caffeine, anticancer drug camptothecin and pesticide fenazaquin.
‘The scope is probably one of the strengths of this paper,’ says Martín Fañanás-Mastral, a sustainable catalysis researcher at the University of Santiago de Compostela in Spain. ‘The functional group tolerance is rather broad and the complexity of molecules that they can use is really impressive. The control of the conditions is really, really important here.’
As the alkane C–H bond is so inert, any products generated by the reaction contain more reactive C–H bonds, he explains. Therefore, Noël’s team employed a large excess of the reagent gases at high pressure to drive the selectivity of these reactions according to probability, rather than reactivity. But, the gaseous excess doesn’t go to waste. ‘If you release the pressure, the gas will segregate and then you can recycle it,’ says Noël.
At present, the team has only studied the process at milligram-scale but is already exploring a kilogram synthesis to demonstrate its potential in industry. ‘The impact is really if you can cross those lines from early drug discovery to process chemistry and we think [this method] can serve both communities,’ says Noël.
However, Fañanás-Mastral cautions that there are still some roadblocks to address before this chemistry is suitable for large-scale deployment. ‘The reagents are very cheap, very abundant, but indeed, the costs associated with this control of conditions, the high pressures, they have to be considered. But I would love to think that industry will implement this in future,’ he says.
Despite this caveat, Fañanás-Mastral is excited by the possibilities presented by this activation of underutilised alkane feedstocks and hopes Noël’s work will inspire the development of new reactions with these gaseous reagents.
References
PC Tiwari et al, ACS Cent. Sci., 2025, DOI: 10.1021/acscentsci.5c00468














No comments yet