A new photocatalytic reaction could become the gold standard for splitting hydrogen gas. The researchers that developed the process showed how the polar, reactive species generated can convert carbon dioxide into useful hydrocarbons with high selectivity – potentially offering an alternative to more energy intensive methods like those based on the famous Fischer–Tropsch process.
The process is ‘a new and efficient mechanism of hydrogen activation’, says project co-leader Nengchao Luo, from the Dalian National Laboratory for Clean Energy in China. ‘This breakthrough could [become] relevant for any hydrogenation reaction,’ he adds.
According to Luo, the new process could find many uses in industry. ‘It’s estimated that 25% of all chemical processes include at least one hydrogenation step,’ he explains.
Luo’s team coated gold nanoparticles with titanium dioxide. When irradiated with near-ultraviolet light, electronic interactions at the gold–titanium oxide interface promote the selective dissociation of hydrogen molecules into reactive fragments. These dissociated hydrogen species readily reduce carbon dioxide into ethane. By combining this process in a flow reactor with an existing ethane dehydrogenation photocatalyst, the researchers created a system that ultimately converts carbon dioxide into ethylene with 99% yield and selectivity.
While ethylene is one of the most versatile chemical feedstocks, with applications across manufacturing, plastics, and agriculture, it is mainly derived from oil and gas and its production is energy intensive, explains Alexandra Barth, an expert in photochemistry at North Carolina State University in the US. ‘However, this tandem strategy for the stepwise reduction and dehydrogenation of carbon dioxide to ethylene uses just light,’ she adds.
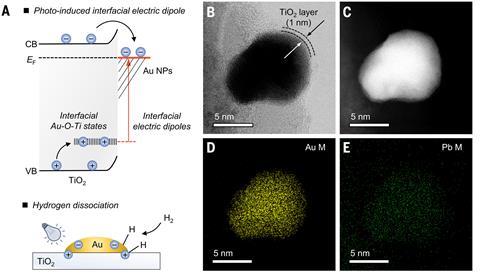
The key to the catalyst’s selectivity lays in its unique structure and mechanistic motif, explains photocatalysis expert Carla Casadevall from the University Rovira i Virgili and the Institute of Chemical Research of Catalonia in Tarragona, Spain. ‘It’s quite uncommon under ambient temperature, typically gold–titanium oxide classic catalysts require extra heating,’ says Casadevall. ‘[Here] the irradiation of UV light generates electric dipoles, trapping the electrons and holes at the interface to subsequently split hydrogen heterolytically,’ she says.
Casadevall points out that the flow chemistry conditions increase the catalysts’ durability, with Luo’s team showing that the reaction could proceed without loss of activity for more than 1500 hours.
‘The catalysts showcase high durability and reproducibility over four independent trials,’ says Barth. However, despite the easy integration into commercial flow reactors, she points out that the high energy UV light sources ‘present poor efficiency of electricity to light conversion’. Another barrier to large-scale applications is the cost of gold. ‘Future goals could include replacing noble metals with earth-abundant materials while minimising efficiency losses,’ she adds.
Casadevall remains optimistic, since ‘the system uses approximately 1% of gold in weight, and showcases long lifetimes, as well as an improved performance as the size of gold catalytic centres decrease.’
Luo notes that his team is also looking into gold-free, greener alternatives such as titanium nitride. However, he explains that the latest work focused on ‘well-known materials to increase our understanding of the reaction mechanism’.
‘The industrial application of photocatalysis is still in its infancy, but [these] preliminary experiments demonstrated economic and technological sustainability,’ he adds.
References
P Jin et al, Science, 2025, DOI: 10.1126/science.adq3445















No comments yet