Be aware – subtle changes can drastically influence the luminescent properties of biological chromophores
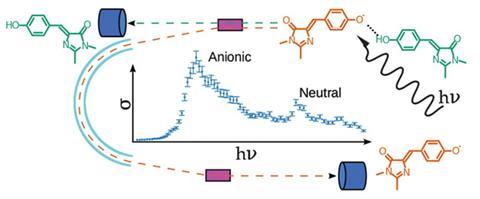
Scientists in Denmark have found that a single hydrogen bond can have a drastic effect on the photophysical properties of molecular chromophores found in fluorescent proteins.1 The newly gained knowledge might help biochemists to develop different coloured variants of these luminescent tools.
Green fluorescent protein (GFP) and its variants are luminescent proteins first isolated from jellyfish. Their luminescence is based on a small biological chromophore in the centre of the protein. GFP plays a vital role in biological imaging and its photophysical properties can be fine-tuned by modifying the chromophore itself or its protein environment. However, our understanding of the specific influence of these different changes is still in its infancy.
Now, the group of Lars Andersen, at Aarhus University, has investigated the colour and photoabsorption of the GFP chromophore in its neutral and anionic states. They conducted the experiments in the gas phase – a challenging task as Andersen explains these ‘rather low ion densities still represent a major difficulty in such experiments’. Andersen’s group was the first to develop the necessary laser-action spectroscopy technique to study the first biological chromophore, the GFP chromophore anion, in vacuum.2 So with this technique in hand, the team decided to investigate one of the most crucial interactions in biological systems: the hydrogen bond. They were surprised to observe that a single hydrogen bond initiated a remarkable 0.5eV shift in the absorption spectrum of the GFP chromophore.
‘This work highlights the sensitivity of the chromophore to subtle changes in its local environment, and might, therefore, help in the continued search for brighter and better GFPs,’ comments Timothy Craggs, a biophysicist from the University of Oxford, UK.
For the future, these results show that a more thorough understanding of biological chromophores from a biophysical point of view might help biochemists to develop new colour variants of GFP in a more rational way. In addition, the research makes us aware that it is vitally important to always consider the effect of external influences such as different media on the hydrogen bonding network and therefore the spectral characteristics of a luminescent protein.
References
- H V Kiefer et al, Phys. Chem. Chem. Phys., 2015, DOI: 10.1039/c5cp02764k (This article is free to access until 14 August 2015.)
- S B Nielsen et al, Phys. Rev. Lett., 2001, 87, 228102 (DOI: 10.1103/PhysRevLett.87.228102)












No comments yet