A totally automated synthesis that allows carbon chains to be grown one atom at a time with control of chain length and stereochemistry has been developed. ‘Mimicking nature’s work, we have instructed a robotic platform to build complex structures in an iterative manner, joining together small units like a Lego set,’ explains Valerio Fasano who works with Varinder Aggarwal at the University of Bristol in the UK. The new system can make six carbon–carbon bonds in a row without human participation. This is the highest number of iterations ever reported for an automated synthesis of carbon chains, says Fasano. ‘We hope to push this record even further.’
Automated synthesis has had a huge impact in the fields of proteomics and genomics, making it easy for non-experts to prepare complex biomolecules such as proteins and nucleic acids. There have also been important advances towards automated polysaccharide synthesis. ‘But when it comes to organic synthesis, many carbon–carbon bond-forming reactions are sensitive to the environment and therefore not suitable for robotic platforms,’ says Fasano. ‘This is particularly true for stereocontrolled reactions that often require working at a temperature of –78°C.’
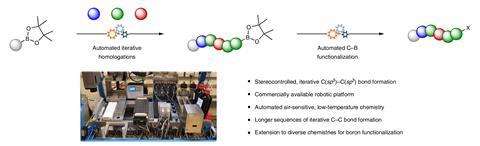
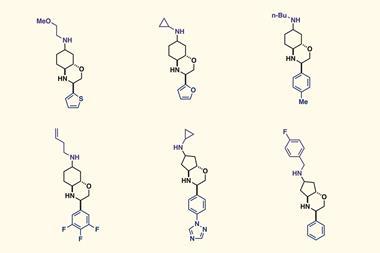
The team programmed a commercial Chemspeed workstation to perform automated homologation reactions of organoboron compounds using carbenoid building blocks. Such reactions don’t only require very low temperatures but also a water- and oxygen-free environment. With the new approach, the scientists were able to make the core fragment of the natural product kalkitoxin – a neurotoxic compound found in cyanobacteria. They also demonstrated the automated conversion of boronic esters into amides. ‘We are now aiming to synthesise other natural products in an automated fashion,’ notes Fasano.
‘The number of carbon–carbon bond-forming steps conducted in the study is most impressive,’ comments James Morken at Boston College in the US. ‘Not only does it point to the efficiency of the homologation reactions, but also to the ability of automated purification techniques to remove byproducts that arise from the individual chemical reactions.’
Daniel Blair of St Jude Children’s Research Hospital says that making molecules has long been the domain of highly trained experts. ‘Aggarwal and colleagues have digitised the process of molecule making by capturing more than a decade’s worth of chemical synthesis experience within an automated process which is reliable and reproducible. They have taught their machine to read between the lines.’
‘This work represents a step forward in how scientists can stitch together molecules in an automated fashion,’ adds Kerry Gilmore from the University of Connecticut. ‘Through the addition of new tools in what type of transformations are possible – in this case the addition of highly reactive chiral compounds – we gain greater access into the areas of chemical space accessible using this iterative approach.’
Morken mentions that the new protocol can be used to make organic molecules that are relevant to drug discovery and biology. ‘It will be fascinating to see what other complicated targets are easily within the practitioner’s reach using fully automated synthesis methods,’ he says.
References
V Fasano et al, Nat. Syn., 2022, DOI: 10.1038/s44160-022-00158-6










No comments yet