Researchers in Japan have created a protecting group that will be useful for making complicated biomolecules. Based on the commonly used fluorenylmethoxycarbonyl (Fmoc) group, the new 2-(2-ethynylphenyl)-2-(5-methylfuran-2-yl)-ethoxycarbonyl (Epoc) group is initially stable in the basic conditions known to cleave Fmoc groups, but it can be activated using gold(iii) catalysis to restore Fmoc-like lability in basic conditions.
When synthesising structurally complex biomolecules such as peptides and oligosaccharides, selectively cleaving protecting groups is crucial when installing different branches and side chains. Striking a balance between stability and ease of removal, however, remains a challenge when developing new protecting groups.
Fmoc groups are easily cleaved in basic conditions due to the presence of an acidic proton on the fluorene ring system, which makes deprotection via an elimination mechanism straightforward. Key to the utility of the Epoc group is that its activated by gold(iii)-catalysed cyclisation. This transformation produces a 4-hydroxyfluorenylmethoxycarbonyl (Hmoc) group, featuring the same acidic proton on the 4-hydroxyfluorene ring system, which can then undergo elimination in the same conditions used to cleave Fmoc groups.
The gold(iii)-catalysed reaction had previously been reported for forming bicyclic phenols from alkynes tethered to furan rings. Katsunori Tanaka at Riken was initially interested in this reaction for synthesising useful compounds such as drugs inside the body, rather than deprotection chemistry. ‘I had been wanting to do different things, wanting to transform functional groups that can be used for in vivo experiments. But my student told me that [using the gold-catalysed reaction] one compound can be transformed to another, which could be used as a protecting group, so that you can deprotect with different conditions at each structure. This is actually a serendipitous finding.’
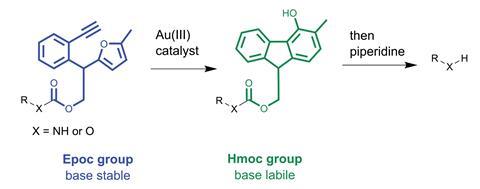
In line with the original direction for this research, Tanaka’s team also successfully transformed the Epoc group into the Hmoc group under the aqueous conditions required for reactions in biological systems. Further studies showed that the Epoc group exhibited a useful level of orthogonality to other protecting groups: being stable in the acidic conditions for Boc group deprotection, in hydrazine used to cleave Dde groups, and in the presence of palladium complexes required to remove Alloc groups. The team demonstrated the practical utility of this deprotection control by synthesising a 13-residue peptide, where a crucial branching step required selective deprotection between Fmoc and Epoc groups. The Epoc group remained stable under Fmoc cleavage conditions, and after the branch had been installed, the Epoc group was successfully activated and cleaved to synthesise the remainder of the peptide chain.
Standard coupling
Protecting compounds with the Epoc group makes use of a standard coupling procedure using the N-hydroxysuccinimide (NHS) derivative of the Epoc group, analogous to protection using Fmoc groups. In addition to stability, and convenient protection and deprotection methods, protecting groups must also be readily available for synthetic chemists to use. Even though it takes six steps to make the Epoc NHS derivative, Tanaka does not consider this to be an insurmountable issue. ‘We can start from very large amounts, so that [the synthesis] is not so tedious. If you wanted to sell this compound as a new reagent, that could be okay.’
‘It is exciting to see that this method expands the impact of gold(iii) catalysis beyond synthetic organic chemistry of small molecules, adding a new tool in the toolbox for the synthesis and modification of biomolecules,’ comments Man Kin Wong, an expert in gold catalysis at The Hong Kong Polytechnic University. ‘Looking forward to seeing the further development of the Epoc group as a versatile synthetic handle for attachment of drug molecules in a controlled release manner.’
References
This article is open access
T Yamamoto, T-C Chang and K Tanaka, Chem. Sci., 2021, DOI: 10.1039/d1sc03125b

![An image showing a [2]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/0/0/4/504004_fja0c01757_0007.jpeg_49119.jpg)









No comments yet