Researchers in Germany have created the first example of a silicon-based protecting group that is removed using visible light. The new protecting group will allow organic and materials chemists to protect alcohols under mild conditions and without needing UV light.
Protecting groups are essential in organic synthesis, allowing modifications to specific positions on a compound. However, the additional steps needed to protect and deprotect an organic compound can impact the atom economy of a synthesis and often require the use of additional solvents or high temperatures.
With sustainability in mind, Armido Studer and two PhD students in his group at the University of Münster set out to develop a protecting group that would combine the benefits of silyl protecting groups with a photochemical removal step. Silyl groups are often used to protect alcohols. By combining them with an acyl silane group, which is known to decompose in blue light, Studer’s group has developed benzoyldiisopropylchlorosilane, which Studer says ‘behaves quite like a bulky silyl group but of course we add on this additional benefit that it is visible light cleavable.’
Petr Klán, an organic chemist from Masaryk University in Czechia, describes the use of visible light as ‘a major advantage’ over the only other photoremovable silicon-based protecting group, the sisyl group, which uses UV light in the deprotection step. This is because as well as removing the protecting group, UV light can also end up cleaving bonds you want to keep. In comparison to existing silicon-based functional groups, benzoyldiisopropylchlorosilane is simpler to use and importantly, contributes to the goal of making organic synthesis more sustainable.
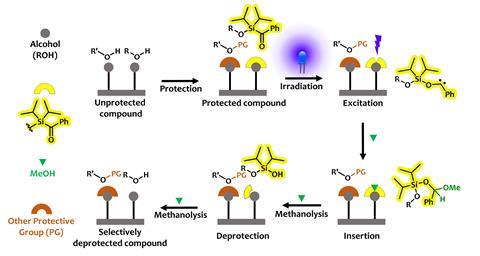
So is this the perfect protecting groups? Benzoyldiisopropylchlorosilane certainly has many positive features, including compatibility with primary, secondary and tertiary alcohols, as well as the ability to be used alongside other protecting groups. However, Klán points out that a major drawback of benzoyldiisopropylchlorosilane is that deprotection must be carried out in methanol, meaning that it is incompatible with the aqueous conditions required by biomolecules.
Although the protecting group may not be suitable for biological uses, it does still have a wide range of potential applications. Hideki Yorimitsu, an organic chemist at Kyoto university in Japan, says, ‘this new photo-cleavable protecting group opens up new possibilities in organic synthesis.’ In addition, Deborah Quaglio, a synthetic organic chemist at Sapienza University of Rome in Italy, states that the new protecting group should lead to ‘a greener process in terms of efficiency and less waste production in a wide range of potential applications’ including nanotechnology and surface modification.
References
This article is open access
F Lind, K Markelova and A Studer, Chem. Sci., 2023, 14, 12615 (DOI: 10.1039/d3sc04975b)



![An image showing a [2]catenane](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/0/0/4/504004_fja0c01757_0007.jpeg_49119.jpg)









No comments yet