A catalyst comprising tiny amounts of platinum dissolved in liquid gallium can be three orders of magnitude more active than solid platinum catalysts for electrochemical methanol oxidation, researchers have shown.
In the quest to maximize atom economy in precious metal catalysts, the ultimate limit is often achieved by single atomic dispersion of the metal’s active sites within a matrix. One challenge of single atom catalysts is to prevent their active sites from deactivating when coming into contact with catalyst poisons such as carbon monoxide. Being a liquid, gallium can avoid this by making the active sites mobile.
In 2017, researchers showed how palladium dispersed in liquid gallium adsorbed on a porous glass surface made for a poisoning-resistant butane dehydrogenation catalyst that outperformed solid catalysts above 200°C. Gallium, with a melting point of just 29.7°C, helped bring the palladium’s melting point down from over 1000°C so that it was liquid at the reaction temperature. But the alloy would still have been solid near room temperature.
Now, researchers have created atomically dispersed platinum by dissolving trace amounts (~0.0001%) of solid beads in liquid gallium at 400°C before cooling it. The catalyst then stays liquid at temperatures as low as 45°C.
The researchers found their catalyst to be active and stable for both oxidation and reduction processes. When used as the anode in electrochemical methanol oxidation, its activity was around 1000 times higher than other platinum–carbon catalysts. It was much less prone to poisoning by the carbon monoxide that is formed as intermediate in the reaction, and binds to and deactivates catalytic sites.
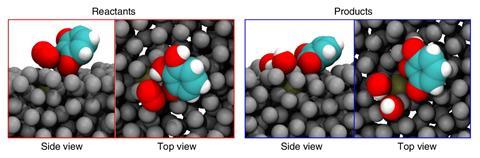
Since gallium can be supercooled to below its melting point, the team could test the catalytic properties of both solid and liquid platinum-doped gallium at 26°C. The supercooled liquid was a far more effective catalyst than the solid. The researchers concluded that the active site’s ability to catalyse a reaction at one point before moving to where the local concentration of remaining reactants is higher greatly enhances the overall kinetics.
The team now hopes to scale up the reaction. ‘One problem is that gallium is approximately one fourth the price of gold at the moment,’ says study leader Kourosh Kalantar-Zadeh from the University of New South Wales in Australia. ‘But the reason is not that gallium is rare – it’s that nobody’s trying to make it cheap.’
They are also investigating extending the technique to other metals. ‘Every time you dissolve any other metal, it’s a pretty unique system by itself,’ says Kalantar-Zadeh’s colleague Md Arifur Rahim. Molecular dynamics simulations suggest that platinum atoms preferentially sit slightly below gallium’s surface, which is key to making it a good catalyst. ‘Some other metals we checked computationally are either protruding from the interface or just underneath and never come into contact with the reactants.’ Though that doesn’t necessarily mean the liquid would be catalytically inactive, he points out.
‘The novelty for me is that it’s a new set of materials that are being explored in catalysis,’ says Bert Weckhuysen of Utrecht University in the Netherlands. He is intrigued by the implications of an immiscible liquid droplet in which the solute and the solvent combine to behave as a catalyst in another liquid. ‘The definition of what is a homogeneous and what is a heterogeneous catalyst becomes a blurred region now for this liquid platinum catalyst, so a lot of the analytical tools that you find in homogeneous catalysis can be applied in heterogeneous catalysis, and interfacial chemistry becomes chemistry and catalysis at the same point.’
References
M A Rahim et al, Nat. Chem., 2022, DOI: 10.1038/s41557-022-00965-6













No comments yet