Catalytic particles offer new cheaper and safer way of storing hydrogen for vehicles
Self-propelled micromotors have for the first time catalysed a reaction that efficiently releases pure hydrogen from a hydrogen-containing salt solution. This feat could offer an alternative and possibly cheaper and safer hydrogen fuel storage system for hydrogen-powered vehicles and devices.
Vehicles based on hydrogen fuel cell technology are already being rolled out, such as Lotus' fuel cell London black cab, and hydrogen filling stations based on compressed gas technology are being built around the world. However, the infrastructure and transport of hydrogen is costly and requires expensive tanks that store the gas at extremely high pressures.
Now, Virendra Singh and colleagues at Joseph Wang's lab at the University of California, San Diego, US, have developed an efficient way to obtain hydrogen from a solution of sodium borohydride. This would allow hydrogen to be generated on-site or on-board a vehicle to power it.
Previously, platinum and other noble metals have been shown to be the fastest at catalysing the release of hydrogen from sodium borohydride. However, films of these metals embedded into supports have run into problems, including catalyst deactivation or destabilisation.
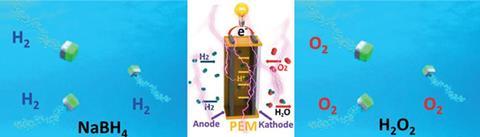
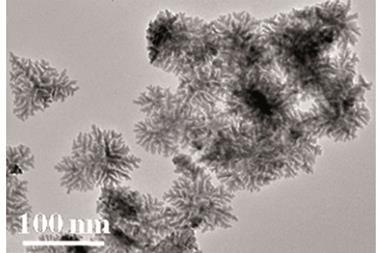
The team made Janus particles with one face made from platinum black and the other titanium. The platinum side had properties that optimised it for the catalytic decomposition of sodium borohydride into hydrogen, which subsequently propelled the particles. Since the titanium side was inert, the particles had an asymmetry that allowed them to move directionally, which enhanced mixing leading to rapid hydrogen evolution.
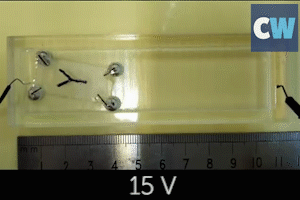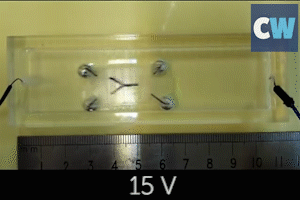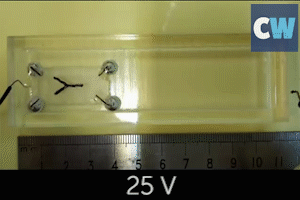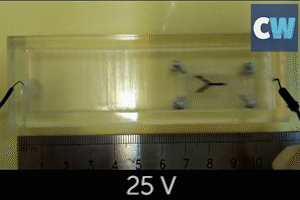
Following this, the team successfully used their catalytic micromotors to release hydrogen and oxygen to power a small fuel cell model car. In this instance, sodium borohydride and hydrogen peroxide were the liquid fuel sources.
However, John Turner at the US government's National Renewable Energy Laboratory has reservations. 'The odds of another hydrogen storage technology supplanting compressed gas are essentially zero,' he says. 'In addition, although sodium borohydride carries a lot of hydrogen, to be practical, one must be able to recycle the by-products back into sodium borohydride. Without a recycle technology this has no value for vehicles.'
Singh agrees on the last point, but argues that sodium borohydride recycling has become an active research topic in recent years. 'While not yet a practical technology, further research could make sodium borohydride recycling possible on a large scale,’ Singh says. ‘The use of renewable energy technology in the recycling process could make this a very favorable process since all of the reactants necessary can be obtained from the spent fuel.’
References
V V Singh et al, Angew. Chem., Int. Ed., 2015, DOI: 10.1002/anie.201501971









No comments yet