New catalyst unleashes more hydrogen for ammonia borane-powered fuel cells
Scientists are a step closer to ammonia borane-powered fuel cells thanks to a ruthenium catalyst that yields an unprecedented amount of hydrogen.
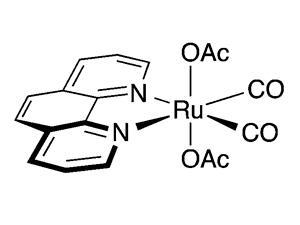
In theory, each molecule of ammonia borane, H3NBH3, can release three hydrogen molecules. This high hydrogen density makes ammonia borane an ideal fuel cell material. In 2011, a team based at the University of Southern California, US, developed a ruthenium catalyst that could liberate more than two of these hydrogen molecules under mild conditions. Led by Travis Williams, the team has now developed a catalyst to release almost all available hydrogen.
Borazine results from ammonia borane releasing two hydrogen molecules and limits a fuel cell’s capacity. Borazine can also poison fuel cells by deactivating the catalyst, and is resistant to further hydrogen release. To tackle these issues, the team adapted a square planar ruthenium catalyst with two acetate ligands. The catalyst produces 2.7 equivalents of molecular hydrogen from ammonia borane. It can be recycled multiple times and is air and water stable. 11B NMR studies revealed that, after two hydrogen molecules emerged, the borazine polymerised, producing polyborazylene and more hydrogen. Thus, borazine formation no longer limits ammonia borane’s hydrogen releasing potential.
References
This article is free to access until 08 June 2016
X Zhang, L Kam and T J Williams, Dalton Trans., 2016, DOI: 10.1039/c6dt00604c











No comments yet