High throughput method could speed drug discovery and cut down use of costly compounds
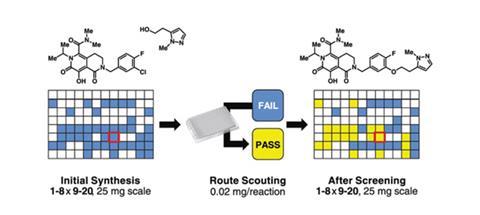
Chemists at pharma giant Merck have conducted over 1500 chemistry experiments in under a day thanks to a miniaturised, high throughput automation platform they developed for identifying how synthetic molecules react under various conditions. The work could speed up drug discovery and provide chemists with a tool kit to explore new medicinal compounds.
A chemist is lucky if they can run 96 reactions in a day
The discovery of drug leads involves synthesising complex molecules and then screening them to identify how they react under various conditions including temperature and concentration. However, a typical screen might require 10mg of a compound to get just one data point, while state-of-the-art methods achieve the same with 1mg of material. Not only is this time consuming, but every milligram is precious in medicinal chemistry and the substrates needed to synthesise complex molecules are invariably in short supply.
Frustrated by these problems, which mean many molecules designed at Merck never get made or tested, Tim Cernak, Spencer Dreher and colleagues at Merck Research Laboratories in Rahway in the US, have found a solution. They combined the robotics used in biotechnology with high throughput mass spectrometry techniques to produce between 50 and 500 times more reaction data than existing methods. The team demonstrated their nanomole-scale method could execute 1536 chemistry experiments in less than a day with as little as 20µg of material per reaction.
‘We are excited about how this technique could encourage the use of new chemistries in drug discovery,’ says Dreher. ‘Medicinal chemists tend to steer towards the reactions they can trust as there’s little time and material for reactions that might fail. We hope that, initially, the adoption of this approach can help medicinal chemists try out a new reaction on their complex substrate without burning up time and material.’
‘You have to make the molecule to test your hypothesis of what it might do so you need a reaction that works – some reactions fail more than 50% of the time on drug-like substrates and that disconnect spurred on this research,’ Dreher adds.
Entering the biochemists’ realm
The team used palladium catalysed cross-coupling reactions as a test case for their miniaturised platform. They developed a reaction system that ran at room temperature in dimethylsulfoxide (DMSO) – a good solvent for dissolving drug molecules – with a combination of ligands and bases. The reaction system was connected to established robotics technology used in biological research. ‘We were so excited to learn how general these conditions are for many flavours of C–N, C–O and C–C cross couplings. There are lots of existing reactions with these properties – room temperature reactivity and homogeneity in a high boiling solvent – but there wasn’t a system like that for palladium cross-coupling,’ says Dreher.
‘Today, biochemists can run upwards of 100,000 experiments in a day on picograms of material per reaction,’ says Cernak. ‘A chemist is lucky if they can run 96 reactions in a day on several milligrams of material per reaction. Our approach is an effort to enter the realm of high throughput experimentation enjoyed by biochemists.’
‘The miniaturisation of reaction screening to the nanomolar scale in a reproducible manner is very impressive and has major implications for reaction development,’ comments Karl Collins, who investigates synthetic chemistry and catalysis at the University of Münster, Germany. ‘The ability to undertake more reactions, more quickly, should hopefully enable researchers to solve challenging synthetic problems in a much shorter timeframe.’ Collins also thinks the ability to screen a large number of reaction permutations to solve problems in reaction development is very promising. ‘Traditional combinatorial methods have clearly highlighted over the years the limitations of varying one parameter at a time.’
Cernak and Dreher hope that chemists will embrace this idea in industry and academia, as well as manufacturers of hardware, software, reagents and consumables. ‘We show how the technique can be used to find productive reaction conditions on complex substrates in a material limited environment, but it’s also a great technique for reaction discovery,’ says Cernak. ‘We’ve used it successfully for inventing completely new reactions; you can generate so much data so quickly it allows you to mix rational design with serendipity.’






No comments yet