High-throughput screening and flow-based scale-up combo identifies chemistry needed to functionalise drug fragments and obtain useful amounts of product
Scientists have coupled high-throughput and flow technology to identify new synthetic routes and reactions for use in drug discovery. The process uses liquid handling robots to run hundreds of reactions simultaneously, identifies successful transformations, and scales up the product by running the successful reactions in a continuous flow reactor. It represents the first time that a high-throughput-based workflow has been used to find a photoredox-mediated C–H functionalisation reaction.1
Currently, one of the leading methods for identifying new drugs is fragment-based drug discovery. Libraries of small molecule fragments are screened against target proteins to identify hits that bind with high affinity. Then, in a process called fragment-to-lead optimisation, these hit molecules are grown in specific directions, to make a larger molecule that has the right functional groups in the right positions to bind more of the target protein surface.
‘Sometimes chemistry exists that allows you to easily grow the fragment hits in these specific directions, but very often what we find is that there isn’t the chemistry available,’ says Chris Johnson, of Astex Pharmaceuticals in Cambridge, UK. Together with his Astex colleague Rachel Grainger, and in collaboration with the University of Cambridge, Johnson set out to develop a process to identify the chemistry needed to functionalise fragments and obtain useful amounts of product.
They focused on the photocatalysed functionalisation of heteroarenes, structures that are present in many common drug compounds but often very difficult to functionalise in fragment-to-lead optimisation. ‘We wanted to take a native fragment and directly elaborate it through carbon—hydrogen bond functionalisation without needing to have a functional group handle. Piperazines and morpholines are often tricky to directly functionalise, so we decided to start with them,’ says Grainger.
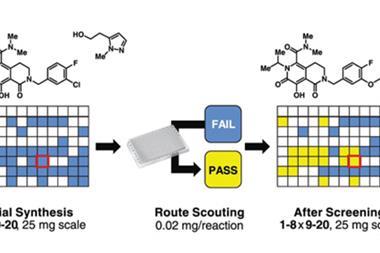
Using the workflow, they screened conditions for over 760 reactions on a nanomolar scale, identifying the best reaction conditions by looking at the relative conversion into product via HPLC. This identified optimal conditions for the photoredox-catalysed coupling of the heteroarenes with carbamyl amines. However, the Astex team haven’t been the only ones working on these reactions, and in the weeks leading up to the publication of their research, two different laboratories also reported their findings on the same types of cross-coupling reaction.2,3
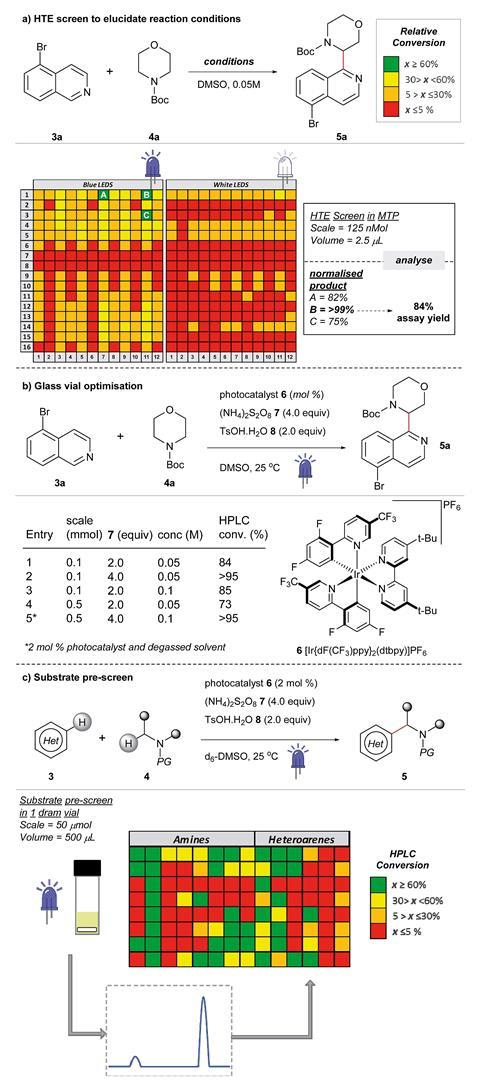
‘Photoredox is a hot area,’ says Johnson. ‘The point of our work is the approach used – taking a fragment hit, developing new chemistry to functionalise it, and not necessarily knowing what precise reaction will be discovered as a result.’
Ian Davies, director of scientific relationships at the Princeton Catalysis Institute, US, which also develops photocatalytic reactions for drug discovery, is impressed by these new methods. ‘Astex has an established track record in using fragment-based drug discovery, and it is exciting to see them extend their chemistry toolbox using the latest methods to improve their fragment-to-lead platform,’ he says.
The group at Astex has now used the workflow to discover other successful functionalisation reactions and say the approach can also be applied to non-fragment-based drug discovery processes, such as late stage functionalisation.
References
1 R Grainger et al,Chem. Sci., 2019, DOI: 10.1039/c8sc04789h (This article is open access.)
2 J Dong et al,Org. Lett., 2018, 20, 5661 (DOI: 10.1021/acs.orglett.8b02389)
3 C Bosset et al,Org. Lett., 2018, 20, 6003 (DOI: 10.1021/acs.orglett.8b00991)








No comments yet