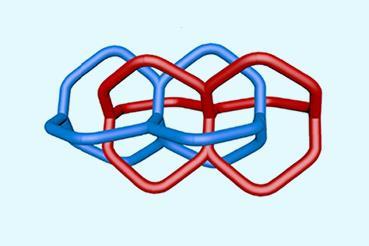
Over six decades after Edel Wasserman from Bell Telephone Laboratories claimed to have made the first mechanically interlocked hydrocarbon rings – without direct evidence – UK chemists have supported his findings.1 For six years David Leigh’s team at the University of Manchester sought to show that Wasserman’s 1960 letter to the Journal of the American Chemical Society was wrong.2 In it, the US chemist claimed the first interlocked pair of rings, which he called a catenane. But after Leigh’s group painstakingly reproduced Wasserman’s work, ‘it must have been formed in his reaction’, he concludes.
Margaret Schott from Northwestern University in Evanston, US, worked with Leigh to access Wasserman’s Bell Labs records. While 1915 Nobel laureate in chemistry, Richard Willstätter, probably first talked about such molecules in a seminar in Zürich, Switzerland sometime between 1906 and 1912, she notes that Wasserman coined the term ‘catenane’.
‘There was much celebration from admirers alongside much scepticism from critics,’ following the 1960 paper’s publication, comments Carson Bruns from the University of Colorado, Boulder, US. ‘I was exposed to scepticism around this seminal catenane almost immediately upon choosing to study mechanical bonds in graduate school , but mainly through informal conversations with peers and mentors.’
Scarce, inconsistent, incredible data
Wasserman’s finding had been viewed so sceptically that Leigh wrote a review paper celebrating the 50th anniversary of catenanes in 2015. In 1964 Arthur Lüttringhaus and Gottfried Schill from the University of Freiburg, Germany, successfully produced catenanes accepted by the whole chemical community. Lüttringhaus and Schill’s key insight was to use structural features that held two open rings near to each other so that they could be more easily linked and closed. It was this paper that Leigh celebrated, yet he was intrigued by Wasserman’s 1960 claim.
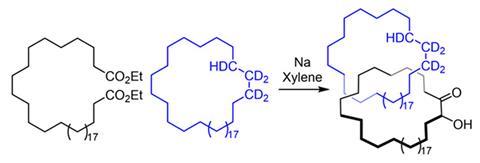
One reason chemists were sceptical is that Wasserman – though it was probably his colleague Louis Barash doing the work – left threading the two rings mostly to chance. He mixed one hydrocarbon ring containing 34 carbon atoms and a few deuterium atoms with a hydrocarbon diester that he cyclised, producing a second ring of the same size. He then purified out the starting materials – including the initial deuterium-containing ring – using chromatography, leaving a product mixture.
Wasserman then used conditions that would break apart the catenane, then claimed that he could detect the deuterium-containing ring in the final product mixture by infrared (IR) spectroscopy. He said this could only be possible if he’d successfully interlinked two rings. Later interpretations of the yields he reported estimated that he made around 1mg of catenane from 10g of deuterated starting material. In a 2019 review,3Reinhard Brückner, also from the University of Freiburg, criticised the ‘scarcity, inconsistency, and even incredibility of pertinent data’. He called the catenane a ‘prophetic compound’ and ‘a structure never established’. ‘I thought “well, we should just check that out,”’ Leigh recalls. ‘How hard can it be? Turns out it’s hard.’
The challenge was a result of the reagents and products Wasserman chose – they were all very fatty. That means that separating them with modern chromatography techniques is difficult. The fatty molecules also don’t travel well through a mass spectrometer. Leigh’s team didn’t focus exclusively on the project either. ‘You don’t want to commit huge amounts of effort to a project where you’re expecting failure,’ Leigh says.
‘Zero doubt’
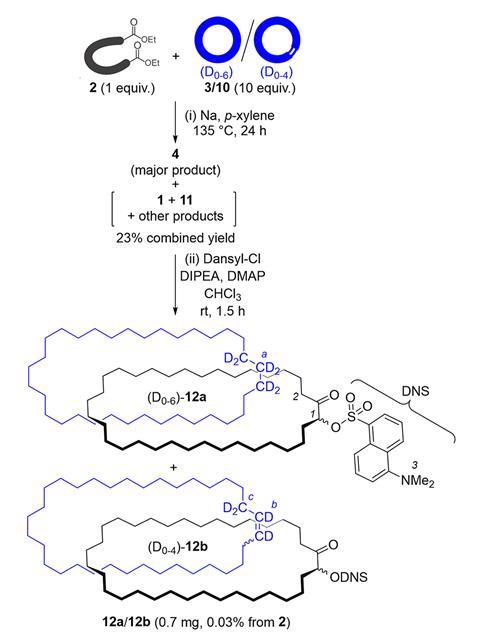
When the Manchester team, mainly PhD student Andrei Baluna, repeated the experiment as Wasserman had done, they couldn’t immediately detect any catenane. Yet they could detect a deuterated compound like Wasserman, though by nuclear magnetic resonance (NMR), having been unable to do so using IR spectroscopy. ‘That was so strange,’ Leigh says. ‘If we hadn’t done that we’d have given up, but this proof of Wasserman’s seemed to be true. If we could figure out where that was coming from, then we could figure out where Wasserman was wrong.’
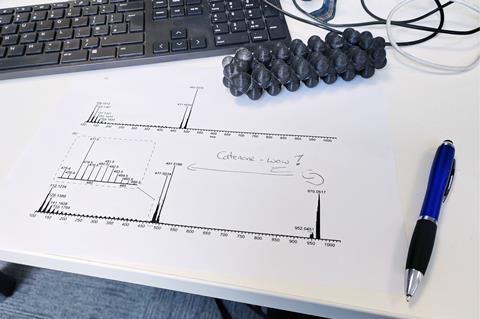
Eventually, the chemists identified traces of the same substance by mass spectrometry. Catenanes have a distinctive signature in this technique, first breaking apart into two separate rings before fragmenting further. ‘As soon as we saw this, we knew it was actually a catenane,’ says Leigh. Applying further modern analytical techniques, they found that they had produced several catenanes, including the one that Wasserman claimed.
Brückner is impressed by the outcome. ‘There’s zero doubt that Leigh has been successful, that the concept of Wasserman accordingly could be realised,’ he tells Chemistry World. He says that it’s ‘conceivable’ that Wasserman made what he claimed. ‘I would go so far and not beyond,’ Brückner adds. He doesn’t believe the Bell Labs researcher proved his mixture contained deuterium, as the amount would have just been five times the naturally-occurring level. ‘I don’t see how IR spectrometers at the time could have possibly detected that,’ says Brückner.
‘I love this paper,’ adds Bruns. ‘It celebrates the mechanical bond through the pioneering work of Wasserman and settles a controversy that has persisted for more than half a century. Even with all of our contemporary methods and tools, it must have been very difficult for the team to isolate and characterise Wasserman’s catenane – a testament to the enormous ambition of Wasserman to undertake it in the 1950s! ’
References
1 A S Baluna et al,J. Am. Chem. Soc., 2023, 145, 9825 (DOI: 10.1021/jacs.3c01939)
2 E Wasserman,J. Am. Chem. Soc., 1960, 82, 4433 (DOI: 10.1021/ja01501a082)
3 R Brückner,Eur. J. Org. Chem., 2019, DOI: 10.1002/ejoc.201900268













No comments yet