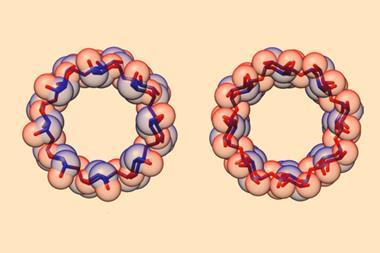
Scientists have found a way to control the chirality of a mechanically interlocked molecule with the help of non-covalent interactions. Using chiral sulfonate anions, a group of researchers who worked with the now late Nobel laureate Fraser Stoddart, was able to bias the equilibrium between the chiral co-conformations of a carefully designed catenane.1 The new strategy allowed them to introduce handedness into the molecule without changing its overall shape. ‘This discovery could inspire advancements in pharmaceuticals and materials science due to the significance of chirality,’ says team member Ruihua Zhang from the University of Hong Kong. ‘Unlike conventional chiral molecules that rely on stereogenic centres, our catenane’s mechanical chirality arises solely from its interlocked topology.’
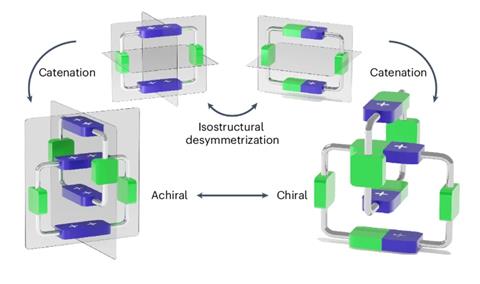
Zhang’s colleague Chun Tang explains that the closely packed structure of the catenane plays a key role in the process. ‘The two rings are not merely entangled; they’re designed to be compact, meaning that they fit tightly together, which reduces random movement – a characteristic feature of interlocked molecules,’ he says. ‘This compact mechanical bond allows us to precisely control the arrangement of the two rings, making them chiral.’

The researchers used their method – called isostructural desymmetrisation – to insert chirality in an originally achiral compound they made in 2013.2 ‘That catenane was constructed from two cyclobis(paraquat-p-phenylene) cyclophanes (CPBQT4+)’, notes Zhang. ‘Each CPBQT4+ ring has three symmetrical planes, giving birth to the catenane HC8+ with two symmetrical planes,’ she adds. To create the chiral molecule, the team slightly modified the ring by replacing two charged nitrogen atoms with two neutral carbon atoms. ‘This subtle change disrupted the ring’s symmetry while preserving the same conformation as the original catenane,’ points out Zhang. ‘The resulting uneven charge distribution and compactness caused the interlocked rings to tangle in a way that produced chirality.’
Stephen Goldup from the University of Birmingham in the UK, who wasn’t involved in the research, explains that the rings can move relative to one another, which leads to two preferred arrangements that have the same energy but opposite chirality. ‘This is what gives rise to the tuneable mechanical stereochemistry,’ he says. ‘Although the molecule exists as a racemic mixture when paired with an achiral anion, when paired with an enantiopure chiral anion the ion pairs are diastereomers and so have different energies.’
‘In our experiments, we used chiral disulfonates to achieve this effect,’ says Tang. The team carried out dynamic NMR spectroscopy measurements and found that there was a barrier of 16.4kcal/mol between the two enantiomeric catenanes. Tang explains that adding the chiral species to the system influences the equilibrium between the two co-conformations. ‘The ions bind to the catenane’s charged rings through electric attraction and weak intermolecular forces, favouring one chiral form over the other,’ he notes.
While the approach works in solution too, complete control of chirality can currently only be achieved in the solid state. ‘When the molecule is crystalised with the chiral anion, the crystal packing of one diastereomer is so favourable compared to the other that a single diastereomer of the crystal is formed,’ comments Goldup. ‘This is important because although mechanically chiral molecules with fixed stereochemistry have been shown to have exciting potential applications in areas like catalysis and chiral emission, making them enantiopure is still difficult.’
The scientists say that the new strategy could be used to design other chiral catenanes and rotaxanes, but scaling up the synthesis remains challenging.
References
1 C Tang et al, Nat. Synth., 2025, DOI: 10.1038/s44160-025-00781-z
2 JC Barnes et al, Science, 2013, 339, 429 (DOI: 10.1126/science.1228429)








No comments yet