The 200-year-old haloform reaction has been given a modern makeover. Using rigorous mechanistic studies to inform their strategy, researchers at the University of Bristol drove this reaction to accept secondary alcohols in stochiometric quantities, something never attempted in the reaction’s history.
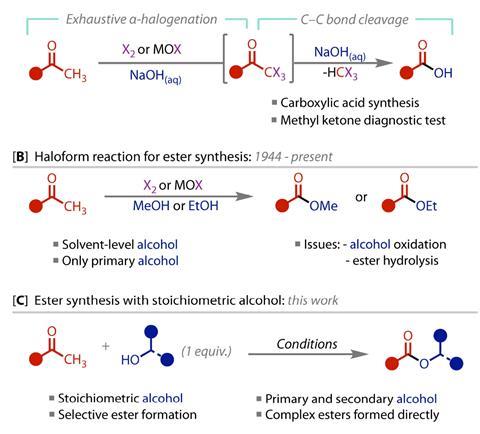
First discovered in 1822, the haloform reaction converts methyl ketones into carboxylic acids or esters, forming an insoluble haloform as a byproduct. ‘The methyl protons are acidified by virtue of being next to the carbonyl which essentially means that the methyl group CH3 can be converted into CX3, the trihalomethyl, which is a leaving group,’ explains Liam Ball, a physical organic chemist at the University of Nottingham who wasn’t involved with the new work. ‘This CX3 group can then be substituted by either water or alcohol at the carbonyl to make a new C–OH or C–OR bond.’ Both mild and reliable, the reaction became an industrial staple for the synthesis of carboxylic acids and methyl esters, but the requirement for solvent quantities of alcohol limited its application in the synthesis of more complex products.
‘We were very surprised to discover that no one had actually used more complex alcohols in this reaction,’ says study author Alastair Lennox. ‘So we set out to discover why that was and whether we could use that knowledge to expand the scope of this reaction.’ The original reaction uses a combination of aqueous bases to generate the trihalogenated leaving group, but subsequent competition of hydroxide ions from this mixture with the intended nucleophile means a vast excess of alcohol is required to favour formation of the ester over the carboxylic acid.
Seeking to eliminate this competing reaction, Lennox’s team began exploring alternative non-aqueous reagents for the initial iodination step, finally settling on the organic base DBU. With these dry conditions, the team could reduce the amount of alcohol to just one equivalent, facilitating the reaction with a range of non-solvent primary alcohols for the first time.
However, the corresponding reactions with secondary alcohols failed to reach completion under these conditions so the team commenced detailed mechanistic studies to identify the problem step. ‘The headline from those studies is that the iodination steps are reversible. Previously that had not been documented,’ says Lennox.
Kinetic experiments revealed the formation of the trihalo leaving group occurs in three reversible steps. The equilibrium favours the product in the first two stages but the final step, which forms the trihalogenated compound from the dihalo intermediate, is not favoured, with a competing side product dominating the equilibrium. ‘The reason that is important is because with the primary alcohol the substitution is very rapid so you don’t observe this reversibility. Whereas, with the secondary alcohols, because the substitution is significantly slower, we see that equilibrium at play,’ explains Lennox.
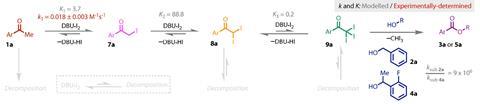
This deep mechanistic insight was the crucial missing piece which enabled the team to incorporate a diverse panel of secondary alcohols into the reaction. ‘In the end, the solution was very simple. We just added more DBU and iodine and that pushed the equilibrium via Le Chatelier’s principle towards the triiodo compound,’ Lennox explains. ‘At a higher concentration, the secondary alcohol could now react with it in more meaningful rates.’
The team’s thorough approach particularly impressed Ball. ‘This could be really beneficial to complex molecule synthesis, both in academia and industry, by enabling the use of more valuable alcohols in this reaction,’ he says. ‘I think the next step has to be extending this to tertiary alcohols too.’
Lennox is eager to exploit the possibilities suggested by these mechanistic insights and the team intend to investigate different nucleophiles as a method to generate other complex molecules from methyl ketones. ‘I think this could really open up new avenues in the formation of different complex products,’ he says.
References
AC Rowett et al, Angew. Chem., Int. Ed., 2024, DOI: 10.1002/anie.202400570














No comments yet