A combination of microdroplet and plasma chemistry has enabled the selective, uncatalysed alkylation of primary amines at an accelerated rate. The US-based team behind the work say that the process offers a greener way to access N-alkylated compounds.
C–N bonds are ubiquitous throughout organic chemistry and reactions that form this linkage are vital tools for synthetic chemists. Existing methods based on catalysis and substitution reactions such as hydroamination are notoriously unsustainable, often requiring high temperatures, rare metals , or forming problematic side products. Some researchers believe that non-thermal plasma chemistry offers a greener alternative. The technique involves generating high-energy particles in the gas phase by passing them through an electric field. The reactant particles are sufficiently energised to undergo reaction without a catalyst or high temperatures, but the process is limited by poor selectivity and scalability.
Now, Abraham Badu-Tawiah at Ohio State University has combined the non-thermal plasma technique with microdroplet chemistry to overcome these limitations. ‘Microdroplets can be envisioned as powerful tiny chemical reactors for accelerating many reactions,’ explains Shibdas Banerjee, a mechanistic organic chemist at the Indian Institute of Science Education and Research, Tirupati, who wasn’t involved in the project. ‘They may render possible a new or unusual reaction, impossible to achieve in the analogous bulk medium.’
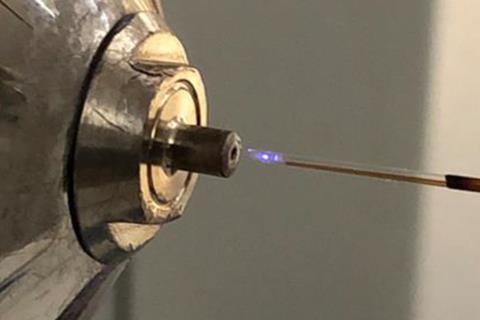
In the new work, a high voltage is applied over an amine reagent as it travels through an electrospray emitter. This generates a plasma microdroplet discharge which rapidly reacts to form the N-alkylated product molecules at room temperature. By controlling both the concentration of the amine reagent, and the timescale of reagent activation in the electrospray emitter, the team were able to select for single or double N-alkylated products.
Badu-Tawiah’s team devised a series of analytical experiments to determine a plausible mechanism for the reaction. These experiments suggest that the amine reagent, which is present as a charged species in the gas phase, is deprotonated by extremely basic reactive oxygen species that are generated in-situ by the electrospray system. High-energy collisions then deaminate a proportion of the now-neutral amine, producing a stable cation which immediately reacts with other neutral amine molecules to produce an alkylated product.
The researchers note that while plasma chemistry traditionally generates gas-phase products, combining the method with microdroplet techniques allows collection of products in the liquid phase, which could enable compounds to be made on preparative scale.
This fusion of techniques has impressed others within the field. ‘This work is excellent,’ comments Elumalai Gnanamani, an expert in microdroplet chemistry, based at the Indian Institute of Technology, Roorkee. ‘The rate of the reaction has increased several-fold and looking forwards, industries may adopt this technique to carry out other reactions.’
‘This is a new finding with enormous promise to explore further such plasma microdroplet systems in performing green synthesis of value-added chemicals,’ says Banerjee. ‘Although this is a proof-of-concept study, it offers opportunities for further developments in the scaling-up process and I am sure this work will get wide attention from the organic chemistry community.’
References
A J Grooms, A N Nordmann and A K Badu-Tawiah, Angew. Chem. Int. Ed., 2023, DOI: 10.1002/anie.202311100















No comments yet