An unusual ‘quasi-benzyne’ radical anion generated from water microdroplets could represent a new substrate class in catalyst-free Diels–Alder reactions. The easy formation of this versatile intermediate and other similar species could provide an alternative strategy for green synthesis of high-value chemicals.
Water microdroplets are opening up exciting new possibilities for organic chemists. They exhibit a surprising range of reactivity not observed in bulk solutions and can accelerate many chemical processes, from cross-couplings to oxidation. Researchers still don’t fully understand the exact mechanism behind these intriguing observations, but it’s thought that electronic effects arising from the droplets’ large surface area influence their behaviour. ‘It is believed that the extremely high intrinsic electric field at the surface of water microdroplets can strip an electron from OH–, forming an OH radical,’ explains Shibdas Banerjee, a mechanistic organic chemist at the Indian Institute of Science Education and Research, Tirupati. ‘This radical acts as a potent oxidising agent, while the freed electron may reduce another chemical species within the microdroplet.’
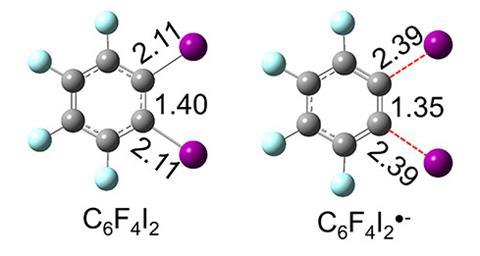
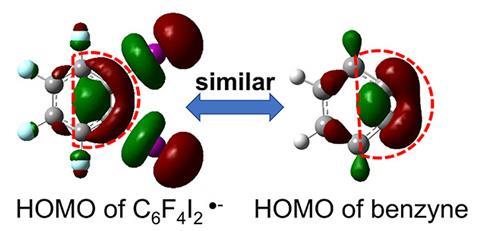
Using this reducing power, Xinxing Zhang from Nankai University in China and Kit Bowen from Johns Hopkins University, US, sought to explore the potential of water microdroplet chemistry as a tool to generate exotic anionic intermediates for catalyst-free reactions. The team passed an aqueous solution of ortho-diiodotetrafluorobenzene through a capillary to form a spray of the corresponding radical anion, C6F4I2•–. DFT calculations and photoelectron spectroscopy analysis revealed that this anionic species possesses a shorter and stronger C–C bond than the parent neutral molecule, giving C6F4I2•– benzyne-like properties.
‘For a closed-shell molecule, an excess electron often occupies an antibonding orbital, making the bond order lower and elongating [all] the bonds,’ explains Zhang. ‘But for this radical, the [extra] electron occupies the I–C1–C2–I linkage, which is actually a combination of two C–I σ* antibonding orbitals and a C1–C2 π bonding orbital. As a result, the C1–C2 bond shortens, but the C–I bonds elongate.’
The team dubbed this unusual intermediate ‘quasi-benzyne’ and were eager to further confirm its benzyne-like character by reproducing benzyne’s signature reactivity. Unlike stable benzenes, benzynes, with their strained C–C triple bond, are a common substrate in Diels–Alder reactions which combine electron-rich ‘dienophile’ donors with electron-poor dienes to make six-membered rings. When Zhang’s team sprayed a mixed solution of C6F4I2 and a diene substrate into microdroplets, the expected Diels–Alder product formed in high yield, without requiring an initiating catalyst.
The potential of this approach to facilitate challenging organic reactions under mild and catalyst-free conditions is particularly significant, says Banerjee. ‘The formation of ‘quasi-benzyne’ using just microdroplets is notable because the species is inherently unstable. However, its unexpected stability at the air–water interface offers the potential for synthesising value-added chemicals,’ he adds. ‘Furthermore, this method could represent a green approach to organic synthesis if it proves effective on a larger scale.’
For Zhang’s team, the focus remains on exploring the new reactivity space created by microdroplet chemistry. ‘I always believe that microdroplets can be a great chance for unexpected reactions that are highly different from the bulk, and a great chance for green synthesis,’ he says.
References
H Chen et al, JACS, 2024, DOI: 10.1021/jacs.4c02819














No comments yet