A strategic atom swap solves a longstanding selectivity problem in the synthesis of complex pyrazoles and is something that has been on the wish list of medicinal chemists for some time. The formal sulfur–nitrogen exchange leveraged asymmetry in the starting material to regioselectively install functionality on one of two resonance-related nitrogen atoms. The team believes this broad conceptual approach, reimagining skeletal editing as a tool to solve early-stage selectivity challenges, could be applied to other substrate classes and heteroatom systems.
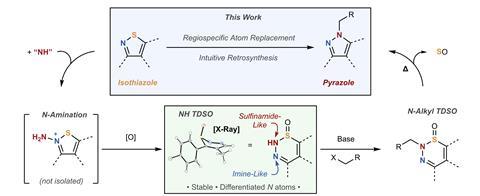
Pyrazole, a simple five-membered heterocycle containing two adjacent nitrogen atoms, is an important motif in pharmaceutical and agrochemical compounds. However, strategies to access complex substituted structures are surprisingly limited, owing to the difficulty of differentiating between the ring’s two heteroatoms. ‘The two nitrogens in the neutral pyrazole are tautomerically related,’ explains co-corresponding author Mark Levin, an organic chemist at the University of Chicago. ‘The hydrogen can hop to either nitrogen and so, even where you have an asymmetric NH-pyrazole, the two nitrogens can adopt either the basic or aromatic position reversibly, so they’re both nucleophilic.’ Consequently, conventional synthetic methods struggle to target a single regioisomer and the resulting mixtures are often challenging to separate, leading to what medicinal chemists call the ‘pyrazole alkylation problem’.
Taking a step back from this troublesome C–N bond formation, Levin and collaborator Christopher Kelly at Johnson & Johnson instead broke the problem down into two manageable parts: desymmetrisation and functionalisation. They began from an asymmetric isothiazole starting material, carrying this asymmetry through an atom exchange reaction to control the position of alkylation in the final product. ‘The idea here is that we’re able to use this atom replacement to control not just the constituent atoms of the ring, but also the substituents that are associated with them,’ explains Levin.
In the first step, the team used a potent electrophilic reagent to aminate the isothiazole at the nitrogen. Subsequent oxidation of the adjacent sulfur then triggered a ring-expanding rearrangement to an unusual six-membered structure called 1,2,3-thiadiazine-S-oxide (TDSO). This unexpectedly stable and isolable intermediate crucially creates a chemical distinction between the two adjacent nitrogen atoms: the nitrogen atom neighbouring the sulfur is sulfonamide-like, making it more acidic, while the distal nitrogen possesses more imine character.
Standard alkylation conditions in the second step therefore selectively target the acidic sulfonamide nitrogen, in most cases giving a single alkylation product. The reaction is then carefully heated to extrude sulfur monoxide, contracting the ring to form a functionalised pyrazole. ‘An interesting point about this process is that it’s agnostic to the alkylation mode so you don’t just have to do SN2 chemistry, you can do SNAr, Mitsunobu…. There’re ways to grow this chemistry and philosophy,’ says Kelly.
Indrajeet Sharma, a synthetic chemist at the University of Oklahoma, was impressed by the simplicity of the solution proposed by Levin and Kelly. ‘A major strength of this study lies in the robustness and practicality of the protocol – it employs manageable reagents and avoids the need for stringent conditions, making it broadly accessible,’ he says. However, the limited substrate scope, restricted to carbon-based groups capable of tolerating the alkylation conditions, may hinder its widespread use as a late-stage functionalisation strategy, he notes.
Similarly, Richmond Sarpong, an organic chemist at the University of California, Berkeley, believes this is an interesting conceptual approach towards a longstanding synthetic problem but that further work is necessary to streamline the method for general use. ‘It is a multi-step process and for this reaction to gain broad utility, the ability to conduct the transformation in the same pot using the same solvent and a single reagent would make it more attractive to medicinal chemists, who are likely to use it,’ he says.
For Levin and Kelly, the reimagining of synthetic strategy is perhaps the most valuable impact of the work and the team is now exploring how this concept could apply to other challenging heterocycles. ‘Up until now, skeletal editing has, for better or for worse, been synonymous with late-stage functionalisation,’ says Levin. ‘But actually, there are other reasons you might want to do skeletal editing. You can solve selectivity challenges and I think that may be an underappreciated aspect of it.’
References
A Fanourakis et al, Nature, 2025, DOI: 10.1038/s41586-025-08951-x














No comments yet