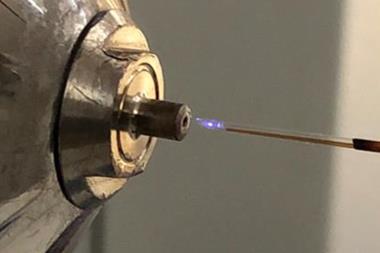
Producing ultrahigh melting point ceramics and alloys for engine and rocket body applications often requires temperatures that are not only ultrahigh but sustained and ideally uniform. A new reactor claims to be able to meet these exacting standards with a setup based on carbon fibres that creates plasmas with temperatures up to 8000K over several cubic centimetres that are also stable for 10 minutes.
The stable plasmas familiar in neon signs and televisions exploit very low pressures, where an applied voltage strips electrons from a gas, which then hurtle towards the positive electrode. However, the low mass of electrons means that even at high velocities they don’t have the momentum to affect chemical reactions. As the velocity of the ions left behind is barely changed, the temperature of the gas itself remains low.
To generate high-temperature plasmas, higher pressures are required so that collisions between the more closely packed ions will increase their velocities. Using a sharp tip that concentrates the electric field around it can create a plasma filament at atmospheric pressure by providing a preferred route for electrons to take – the principle behind lightning rods. However, here the temperature only increases along the filament. The temperature difference between the plasma filament and the gas around it makes these plasmas very unstable.
‘What we do here is we have a combination of both,’ says Liangbing Hu at the University of Maryland. ‘We have tips so we have high temperature, but they support each other so we have stability.’
The carbon advantage

The researchers used carbon fibre felt at either side of their atmospheric pressure plasma cavity. Some of the carbon fibres woven into the felt naturally lie flat while others stand upright. These bundles of short upright fibres are key to plasma stability, effectively providing an array of lightning rods so that the plasma filaments from each coalesce giving a uniform and extremely high temperature.
‘We got lucky,’ Ji-Cheng Zhao, also at Maryland, tells Chemistry World, highlighting the excellent thermal conductivity of carbon fibres, which efficiently conducts the heat away. ‘Even though the plasma temperature is 8000°C we are not burning the tip like crazy – we can sustain the plasma for 10 minutes.’
As Hu points out people have long been aware that a tip helps when creating a plasma. Scientists have also tried creating pillar structures in the hope of generating the effect that the US team has now achieved. However, none have apparently attempted the approach with carbon, which proved to be the magic ingredient. Carbon holds onto electrons much less tightly than metals so that the emission voltage is lower and it has one of the highest melting temperatures for known materials – although even carbon should be burnt away at the ultrahigh plasma temperatures achieved, it survives thanks to its high thermal conductivity. Even rogue long fibres standing perpendicular to the felt surface play a useful role as they initiate the plasma. ‘The critical thing is the use of these carbon arrays of tips,’ says Hu. ‘That basically solved all the problems.’
Lakshminarayana Rao at the Indian Institute of Science, who has also looked into ways of exploiting plasmas but was not involved in this research, highlighted how useful this development could be. ‘Thermal plasma have high energy densities which facilitates extremely high-temperature operations and material processing leading to invention and testing of new materials.’

The researchers have already used the setup to synthesise hafnium carbonitride – ‘the highest melting point material predicted from first principles’ Zhao notes and hence useful for various high-performance engines and even rockets. The researchers were also able to pulse the plasma on and off, so that they could exploit the set up to make an amorphous glass from MgO, despite its very high melting point of 2850°C.
Now they are looking at whether the reactor could be used to destroy so-called ‘forever chemicals’ or PFAS. Although the volume of their reactor was limited by the power supply of the lab, the researchers believe that with more power they can increase it from several square centimetres across, and tens of millimetres deep.
References
H Xie et al, Nature, 2023, 623, 964 (DOI: 10.1038/s41586-023-06694-1)















No comments yet