Researchers in Czechia have observed an unprecedented inverse relationship between temperature and reaction rate for a plasmon-assisted azide–alkyne cycloaddition, achieving a ten-fold increase in efficiency when they cooled the reaction from room temperature to −35°C.
Localised surface plasmon resonances, or plasmons for short, are oscillations of a metal nanostructure’s free electron density. They can be excited by electromagnetic radiation and used to promote a range of chemical transformations. In typical plasmon-assisted catalysis thermal and plasmon effects work synergistically; plasmonic excitation activates the reactant molecule, lowering the activation energy for the desired chemical reaction, and conventional heating overcomes the residual activation barrier. However, when studying a plasmon-assisted azide–alkyne cycloaddition, Oleksiy Lyutakov’s group at the University of Chemistry and Technology, Prague, observed the opposite – an acceleration in the plasmon-assisted reaction when the temperature was lowered.
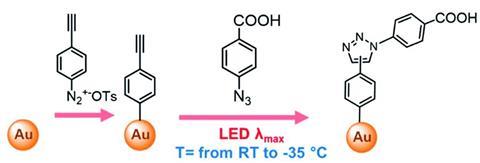
Erik Andris from the Czech Academy of Sciences, who worked on the theoretical parts of the study, attributes this unusual observation to ‘the freezing of plasmon-induced excited intermediate states’. ‘We believe that the catalysis involves the transfer of electronic excitation between the plasmon and surface-bound alkyne reactant, which then reacts with the azide present in the solution.’ In this system, plasmonic effects alone can overcome the reaction’s activation barrier, so heating is not required to promote the reaction. Adris explains that heating is detrimental ‘since it restricts the lifetime of the surface plasmon and leads to faster relaxation of the plasmon-induced excited molecular states’. Therefore, a decrease in temperature extends the lifetime of plasmon-induced excited states, increasing the probability of the desired reaction occurring.
The reaction efficiency was an order of magnitude higher at -35°C than at room temperature. ‘Initially, we expected some acceleration of the reaction rate,’ says Andris. ‘The observed acceleration, however, significantly exceeded the original expectations.’
Emiliano Cortés, who studies plasmonic and photonic chemistry at Ludwig Maximilian University of Munich in Germany, notes that the low-temperature efficiencies achieved here are even higher than those achieved using copper, the best-known heterogeneous catalyst for this click reaction. ‘These findings highlight the exciting opportunities for controlling chemical reactivity by carefully balancing temperature and plasmon excitation.’
Adris says that while it is difficult to draw any far-reaching conclusions at this stage, ‘taking into account the large potential of plasmonics, the ability to carry out reactions at a low temperature – or simply without heating – may turn out to be very interesting.’ This work opens opportunities for controlling product selectivity in plasmon-assisted reactions and for carrying out reactions involving temperature-sensitive reagents. Adris and his team hope to extend this study: ‘If it is possible to repeat the results obtained with other chemical transformations, then, without a doubt, this scientific avenue can become very attractive.’
References
This article is open access
O Guselnikova et al, Chem. Sci., 2021, DOI: 10.1039/d0sc05898j
Additional information








No comments yet