Traditional catalysts can lack both efficiency and selectivity. Tim Wogan explains how plasmons offer the potential to do chemistry with a lighter touch
As the need to wean society off fossil fuels grows ever more intense, the impetus to use renewable sunlight to power the chemical industry grows ever stronger. The electromagnetic waves in sunlight can be many thousands of times the size of an atom, however, which makes coupling sunlight directly into atomic systems inefficient. But plasmonics may offer an elegant solution.
Localised surface plasmon resonances, or plasmons for short, are quantised oscillations of a metal nanostructure’s free electron density. Incoming electromagnetic radiation can excite them through the so-called plasmonic interaction. At the nanostructure’s plasmon resonance frequency (the natural frequency at which free electrons in the nanostructure oscillate), nanoparticles can act as antennas for the electric field, making them extremely efficient absorbers of radiation. Moreover, whereas visible or infrared light has a wavelength of hundreds or even thousands of nanometres, the wavelength of a plasmon can be just a few nanometres.
On the one hand, this allows physicists and electrical engineers to produce much smaller optical circuitry than possible with traditional silica waveguides. On the other, it allows chemists to hugely amplify the interaction between light and matter. This has significantly influenced the study of chemistry through imaging techniques such as surface enhanced Raman spectroscopy (Sers; see Ready for a Raman shift). Plasmons excited on a gold or silver substrate enhance the electromagnetic field a molecule experiences many thousands of times, amplifying weak Raman signals by factors up to 1014. Even single molecules can be detected.
Plasmonics cannot just study molecules, however, it can also influence them – as nanochemists have exploited to control nanoparticle growth since the early 2000s.1 ‘The electromagnetic field of light normally sees the molecule or atom as a point object,’ explains Prashant Jain of the University of Illinois at Urbana–Champaign in the US, an expert in the interaction of light and matter. ‘But when you squeeze the field down to the scale of the architectural features within the molecule, the nature of the interaction completely changes. The molecule can see the electromagnetic field gradient, whereas for regular light it just sees a uniform field. This becomes useful when driving optical processes and transitions that are unknown or even forbidden using regular light–matter interactions.’
Gone in a flash
Once formed, a plasmon decays within femtoseconds. It can do this in two possible ways: the first involves photon emission. As the photon’s frequency is fixed by the electronic energy levels in the material, this effect leads an illuminated object to emit a particular colour of light. This can be seen in the spectacular coloration of antique objects like the late-Roman Lycurgus Cup in the British Museum, which appears green in reflected light but red in transmission because of tiny quantities of silver and gold nanoparticles dispersed in the glass. ‘This developed further in the middle ages,’ says Alberto Naldoni of Palackỳ University in the Czech Republic, ‘Metal oxides and tiny metal particles were added to glass raw materials to give rise to very strong coloration of the finished glass.’

The second plasmon decay channel does not involve light emission. Instead, the energy is released into a high-energy electron–hole pair. In optoelectronics applications of plasmonics, this is considered loss: ‘For 15 years in plasmonics we were working on minimizing the non-radiative decay,’ says theoretical physicist Peter Nordlander of Rice University in Texas. ‘There’s a lot of people doing Raman and waveguiding and subwavelength optical devices – probably the majority – that still want radiative decay and are working with low-loss materials.’ However, this ‘loss’ enables plasmons to drive not just optical transitions – which are effectively instantaneous – but also chemical reactions, which require time for atoms to rearrange themselves.
A catalyst for change
In 2011, building on the work of those nanochemists, surface chemist Suljo Linic and colleagues at the University of Michigan in the US tackled several industrially important reactions such as carbon monoxide oxidation and ethylene epoxidation that normally require high temperatures to proceed quickly. They showed that, if the reactants were adsorbed on a silver nanoparticle surface at moderate temperatures, the reaction could be speeded up significantly by irradiating the surface at the nanoparticle’s plasmon resonance frequency. ‘That provided an initial observation that, if you shine light on a plasmonic metal nanoparticle and expose it to a reactive environment, you can drive chemistry in a way that appeared different than if you just heated the material,’ explains Linic. ‘This led to a field known as plasmonic catalysis. We’ve gone from this to having hundreds – maybe even thousands – of papers published annually in the field.’2
The main advantages of plasmonic catalysis are that it can be highly efficient and selective. In traditional thermal catalysis, the energy supplied to the reactants by heat is divided evenly among all accessible degrees of freedom in the molecules. This wastes energy and may lead to the formation of undesired side-products or even premature degradation of the reaction vessel. The plasmonic interaction, however, is a resonant process: nanoparticles can be designed to absorb energy at specific, sharp resonances, exciting an electron to a precise energy so that it will pass into a specific anti-bonding orbital of a molecule. This can activate the breaking of one bond but leave another, potentially lower-energy, bond untouched.
If you shine light on a plasmonic metal nanoparticle and expose it to a reactive environment, you can drive chemistry
Linic’s group demonstrated an intriguing example of this in 2013. In theory, copper nanoparticles are a good catalyst for the epoxidation of propylene to propylene oxide. In practice, however, they rapidly become oxidised, causing their selectivity to plummet. The researchers found that, under broadband visible light at around five times solar intensity, the selectivity of oxidised copper nanoparticles was recovered from 20% to 50% within two minutes. They proposed that the light had targeted the plasmonic photoreduction of the Cu2O on the surface of the nanoparticles back to the metal, recovering the nanoparticles’ catalytic selectivity to propylene oxide.3
The central problem, however, was that, though these papers had demonstrated the principle of hot-carrier-induced plasmonic chemistry, the reactions were woefully inefficient. ‘The problem is that the only good plasmonic materials we have are gold, silver, aluminium and some alloys – and they are horrible catalysts,’ explains Nordlander. In fact, the very features of the band structures of metals like palladium, platinum and ruthenium that allow them to adsorb reactants and induce redox reactions – making them such effective catalysts – also act to suppress the plasmonic interaction. So what hope is there for efficient plasmonic catalysis?
Sunlight and a big magnifying glass
Ironically, the key to generating hot carriers efficiently lay in abandoning the non-radiative decay mechanism. In 2016, Nordlander and Halas showed that, although plasmonic nanoparticles like gold cannot themselves generate hot carriers efficiently, the electromagnetic field enhancements that prove so useful to researchers in Sers can also excite hot electron–hole pairs in nanoparticles of catalytic metals. The researchers have developed bimetallic ‘antenna–reactor’ particles. The antenna at the centre is designed to undergo a strong plasmon resonance with electromagnetic radiation of the desired frequency. This excites a very strong, highly localised electromagnetic field at the surface of the antenna. Electrons in the reactor particle can be excited by the energy in this field and tunnel between the reactor particle and a molecule adsorbed on its surface. ‘The antenna is just a big magnifying glass that channels energy onto the reactor and creates electron–hole excitations,’ explains Nordlander.
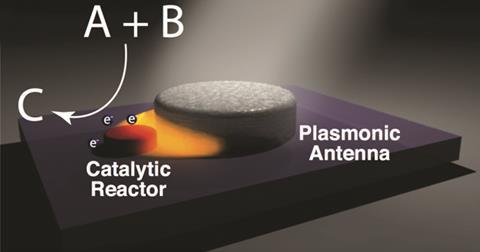
In the 2016 paper, the researchers used aluminium nanocrystals decorated with palladium reactor particles to catalyse the decomposition of H2 and D2. When they irradiated the antenna–reactors with light at the plasmon resonance frequency of the aluminium, the rate of HD production increased even faster than the laser power – a signature of a hot-carrier-driven process.4 The researchers have subsequently extended their antenna–reactor concept to produce plasmonic catalysts for various other reactions. ‘We can tailor the energy of a plasmonic nanostructure to generate carriers of exactly the energy we want for a particular chemical reaction,’ says Nordlander. In October 2018, the researchers demonstrated that copper–ruthenium antenna–reactors could make ammonia decomposition – which may prove important for safe release of hydrogen in fuel tanks – up to 177 times faster.5 Linic’s group in Michigan has also worked with bimetallic plasmonic nanoparticles. In 2017, they found that, when they attempted to catalyse the oxidation of carbon monoxide using light at the plasmon resonance frequency of silver, uncoated 75nm silver nanoparticles just scattered the photons and the reaction achieved only 10% selectivity. When they added just 1nm of platinum to the surface, however, the nanoparticles absorbed most of the photons and channelled them into the oxidation, achieving 70% selectivity at much lower temperatures.6
The holy grail for plasmonics researchers is to drive chemical reactions using sunlight. This, explains Nordlander, would allow the chemical industry to use solar energy far more efficiently than driving conventional chemical processes using solar-generated electricity. ‘If you have to use the electricity from a solar cell with, say, 15% efficiency to drive a conventional catalytic process, and the efficiency of that is small, you are dealing with about 5% efficiency overall by the time you’ve done the two conversions – from light to electricity and from electricity to heat and pressure,’ he says. ‘The efficiency we demonstrated in the 2018 paper was 18%!’
One downside of relying purely on direct solar irradiation, says Nordlander, is that industry would be reluctant to depend on the Sun shining. ‘If you’re investing in capital equipment, you need to be able to sustain the production at night,’ he explains. ‘What has changed the field is the development of LED light sources, which can have a quantum efficiency of 85%. I think this is the reason we see so many people coming into the field now.’
Halas and Nordlander have now co-founded the start-up company Syzygy Plasmonics, whose mission is ‘to utilise breakthroughs in plasmonics to drive sustainability in the energy and chemical industries’. In astronomy, a syzygy is a straight line configuration of multiple celestial bodies – such as the Sun, Moon and Earth during an eclipse. The quantities in question for the new company are ‘energy, technology and sustainability’. As its first reaction, the company intends to commercialise the photocatalytic steam reforming of methane to generate sustainable hydrogen from natural gas.
The (sustainably driven) road ahead
Significant challenges remain for plasmonic chemistry. Experiments so far have usually been performed with monochromatic laser light at the resonant frequency of the plasmonic nanoparticles. The Sun, however, is an inherently broadband light source, so exploiting solar energy efficiently is likely to require using the energy in a wide range of wavelengths. ‘The nice thing with plasmonic nanostructures is that we can tune their resonant energy,’ says Nordlander. However, particles that could excite carriers into the same electronic orbital of a molecule after absorbing different wavelengths of light would effectively be different antenna–reactors. ‘You could do it in a tandem situation or in a mixture, but you’d have to go beyond what we’re doing now,’ he explains.
A further complication is that hot electron–hole pairs can recombine within a fraction of a second, producing either heat or light. ‘In hot-electron chemistry, the electron transfers its kinetic energy to an adsorbate and then returns back to the metal,’ explains Jain. ‘It’s not an irreversible charge extraction process.’ The problem, he explains, is that many of the most important reactions in chemistry, such as artificial photosynthesis and nitrogen fixation, require the movement of multiple electrons. These reactions are therefore kinetically disfavoured.
I see this as a possibility to remediate the disaster that is awaiting Earth unless we do something about greenhouse emissions
Jain’s group has developed a method to permanently extract the electron from the nanoparticle, producing a stable, charge-separated state. In 2018, they showed that adding a hole scavenger such as isopropanol could neutralise the nanoparticle. This allowed the researchers to perform multi-electron reduction of carbon dioxide under visible light.7 Ultimately, says Jain, the researchers would like to avoid adding an extra hole scavenger by creating holes energetic enough to oxidise water. ‘Now you would have a process which reduces carbon dioxide using electrons and oxidises water using the holes,’ says Jain. ‘Overall you would be doing a full, energetically uphill photoredox reaction that combines carbon dioxide and water and produces hydrocarbon fuels.’
Unfortunately, the metals commonly used for plasmonic catalysts – silver and gold – have such low melting points they are unsuitable for many proposed industrial applications of plasmonics that require operation at high temperatures. Among these are solar thermophotovoltaics, solar thermal water splitting for hydrogen production and some indirect plasmonic catalysis that requires extreme local heating of the nanoparticle rather than direct electron transfer. Naldoni, together with Vladimir Shalaev of Purdue University in Indiana, US, is therefore working to develop new ‘refractory plasmonic’ materials such as titanium nitride and zirconium nitride, which both have melting points approaching 3000°C.8 ‘I think titanium nitride could be a real game changer in the near future,’ says Naldoni. Linic is cautious about the potential of plasmonics in the near-term: ‘Any field is going to go through a phase of excitement and maybe overpromise before a steady state is reached eventually,’ he says. ‘Commercial application of plasmonic chemistry is not just about the underlying physical chemistry – it’s also about whether we can design reactors that are efficient in harvesting energy, that have low enough volumes to allow high volumetric flow rates.’
Nordlander agrees: ‘It may be 20 years before you see industrial chemistry on a large scale.’ Nevertheless, he says, ‘I see this as a possibility to remediate the disaster that is awaiting Earth unless we do something about greenhouse emissions. If there’s a 1% chance we’ll succeed, it’s 100% worth doing.’
Tim Wogan is a science writer based in London, United Kingdom
Article updated 23 Jan 2019 to include earlier nanoparticle growth work and upate reference 7
References
1 R Jin et al, Science, 2001, 294, 1901 (DOI: 10.1126/science.1066541)
2 P Christopher, H Xin and S Linic, Nat. Chem., 2011, 3, 467 (DOI: 10.1038/nchem.1032)
3 A Marimuthu, J Zhang and S Linic, Science, 2013, 339, 1590 (DOI: 10.1126/science.1231631)
4 D F Swearer et al, Proc. Natl Acad. Sci. USA, 2016, 113, 8916 (DOI: 10.1073/pnas.1609769113)
5 L Zhou et al, Science, 2018, 362, 69 (DOI: 10.1126/science.aat6967)
6 U Aslam, S Chavez and S Linic, Nat. Nanotechnol., 2017, 12, 1000 (DOI: 10.1038/nnano.2017.131)
7 S Yu et al, Nano Lett., 2018, 18, 2189 (DOI: 10.1021/acs.nanolett.7b05410)
8 U Guler, A Boltasseva and V M Shalaev, Science, 2014, 344, 263 (DOI: 10.1126/science.1252722)










No comments yet