First produced by French chemist Louis Jacques Thénard in 1818, hydrogen peroxide (H2O2) has many uses. The chemical industry produces over 5 million tonnes of H2O2 per year, for products such as disinfectants, bleaching agents and even rocket fuel. Nearly half of global H2O2 production finds its way back into other chemical processes, adding value to numerous chemicals through oxidation.

Today, the anthraquinone process – developed by BASF in 1939 – is the near-exclusive way of producing H2O2. Hydrogen reduces anthraquinone – a cheap, widely available organic reagent – in the presence of a palladium catalyst. Oxidation of the resulting diol with dioxygen regenerates anthraquinone, producing H2O2 as a by-product. Aside from its ease of synthesis, the only by-product of H2O2 oxidation is water, limiting environmental impacts.
Make it where it’s needed
To reduce transport and storage costs, suppliers concentrate H2O2 to around 30–70%, whereas most users require concentrations below 10%. This wastes energy, uses large amounts of water to re-dilute appropriately and raises concerns about working with such concentrated oxidant. ‘Minimising the use of finite resources and preventing the formation of pollutants must be a major focus [going forward],’ says Richard Lewis, entrepreneurial lead of Hydro-Oxy.
Housed within the Cardiff Catalysis Institute, UK, Hydro-Oxy aims to overcome these issues by generating H2O2 in-situ. Its technology uses a palladium-based composite catalyst to convert dilute concentrations of hydrogen and oxygen – of similar levels to that generated by electrochemical water-splitting – into hydrogen peroxide. Doping the catalyst with additional metals such as gold and platinum can boost the activity and stability of the catalyst.
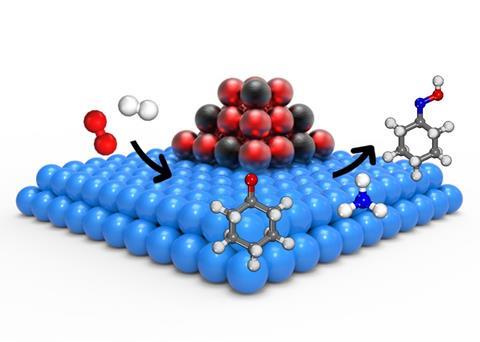
The company has primarily used the technology – with the addition of ammonia – to synthesise cyclohexanone oxime, a key intermediate in forming caprolactam, the monomer for polyamide-6. The process can be adapted to other commodity chemicals – including propylene oxide, methanol and phenol. Hydro-oxy’s technology generates yields approaching 100%, lowers materials costs by up to 15% and reduces energy consumption by up to 30%. Lewis explains that this makes in-situ hydrogen peroxide production ‘far more economically competitive to existing industrial processes’.
‘The benefit of our system is that it can be incorporated into existing [industrial] infrastructure, which lowers the barrier to entry,’ says Lewis. Under industrial conditions using concentrated reagents, the catalyst can last for over 100 hours in stream and has an overall lifetime of around 18 months.
‘We’ve demonstrated in house that we can achieve a multi-litre scale and we’re currently starting experiments at a 20-litre scale with partners based in Beijing,’ says Lewis. He adds that this is possible due to the support from industrial partners, such as Johnson Matthey.
Organic adaptations
‘Lots of water in an organic process is not good news. Many organic molecules are not soluble in water… or they react,’ explains James Clark, co-founder of Addible. He adds that industry has circumnavigated this issue using biphasic systems, where organic substrates react with hydrogen peroxide at the boundary between organic and aqueous solutions. Clark explains that a combination of a tungsten-based catalyst and a phase transfer catalyst for improved communication between the layers leads to ‘a pretty complicated soup, which is far from ideal’.

Addible is hoping to simplify the process by taking H2O2 out of water. The company – a spin-out from the University of York, UK – had initially identified tetramethyloxolane (TMO) as a green solvent that could replace toluene, which is carcinogenic. TMO is similar to tetrahydrofuran, but with four methyl groups blocking the carbons adjacent to the oxygen atom.
Computational characterisation revealed that TMO is very good at hydrogen bonding. ‘If you take standard aqueous hydrogen peroxide and shake it up with TMO, a hydrogen bonded complex forms,’ says Clark. ‘It’s a significant entity with quite strong bonding.’ The complex – known as TMO2 – is stable over long periods and solubilises most organic compounds. Addible can currently produce TMO2 on a 10 litre scale, and its parent solvent TMO on a 1 tonne scale.
TMO2 has so far oxidised a wide range of compounds in non-aqueous conditions, including alkenes, aldehydes, organic sulfides and amines. Clark notes that the activity is somewhat lower than other methods, but addition of a non-metal catalyst improves the system. While Addible’s technology doesn’t remove the need to produce H2O2 in the first place, it solves an existing problem within industry, making things safer, more effective and reduces overall costs.
Other chemists have previously overcome the issue of water in such reactions by using organic peracids – made by oxidising carboxylic acids. Such molecules are common, but their instability can make them dangerous, with some restrictions now limiting their transport. Carboxylic acids regenerated as unwanted side products can also create excess waste.
The company has worked with numerous companies in the green chemistry space to finance its technology. Aside from oxidation of small molecules, Addible is now looking to expand into recycling rubber tyres and other polymers found in textiles and plastic packaging.
‘Imagine a system where you’re making hydrogen peroxide using a modern method, like electrochemistry, or photocatalysis, generating it in-situ, and the TMO2 is extracting it as it’s formed,’ says Clark. ‘Then you have a really nice continuous system.’
Hydro-Oxy
Founded: 2024
Location: Cardiff, UK
Employees: 5
Origin: Housed within the Cardiff Catalysis Institute
Hydro-oxy recently won the ‘enabling technologies’ category at the RSC’s emerging technology competition.
Addible:
Founded: 2020
Location: Dublin, Ireland
Employees: 7
Origin: Spin-out from University of York, UK















No comments yet