Self-illuminating nanoparticles shine a light on cancer
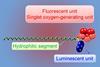
Tumours trigger luminescence for imaging, then production of singlet oxygen to kill cancer cells
A new kind of luminescent nanoparticle equipped to highlight inflammation, track disease progression and combat cancerous tumours does so without the need for outside light, offering an improved method of spotting and treating inflammatory-based diseases and disorders.
Nanoparticles as probes for luminescence imaging are a simple, fast and cheap way of detecting inflammation, a common hallmark of cancer. Despite this, several key issues still remain with current nanoprobes. They tend not to be easily absorbed into and distributed through tumour tissue after intravenous delivery, which is fine for laboratory analysis, but less useful for diagnosis and treatment in vivo. They are also usually activated by an external light, which limits their use to tumours close to the skin surface.
