First system to work at low temperatures that doesn’t generate nitrous oxide
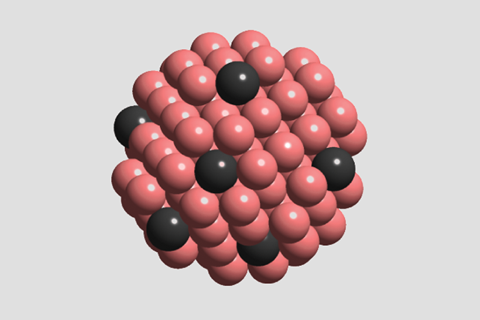
Lone palladium atoms dispersed in copper can reduce nitric oxide at low temperatures without producing the air pollutant nitrous oxide. Compared to current systems, the catalyst could minimise precious metal use and decrease exhaust emissions.
Petrol and diesel engines generate nitric oxide (NO) and nitrogen dioxide (NO2) – collectively known as NOx. Current catalytic converters reduce NOx to nitrogen, but only do this efficiently once warmed up. This means most pollution escapes in the five minutes after drivers turn the ignition. Catalytic converters also struggle to operate at low temperatures without releasing some nitrous oxide (N2O), instead of just nitrogen, because converting nitrous oxide to nitrogen requires temperatures over 300°C.
The catalytic system in this research is the first to completely remove NOx below 200°C. Led by Shinya Furukawa, the team based at Hokkaido University in Japan managed this whilst simultaneously using 90% less palladium than a non-alloyed palladium catalyst.
This alloying could lead to better performance than that from just single palladium atoms
Counter-intuitively, reducing the palladium content improved both the nitric oxide conversion and the catalyst’s selectivity for generating nitrogen, rather than nitrous oxide. Michail Stamatakis, whose team at University College London, UK, uses computational techniques to understand single atom alloys and precious metal catalysts for emissions control, says the catalytic system could save significant quantities of precious metals.
The team investigated the catalyst mechanistically using kinetic analysis and density functional theory. Relative to pure copper or palladium catalysts, nitric oxide reduction on the single atom alloy catalyst required less energy, making it more efficient. The researchers’ investigations also discovered the system successfully eliminated nitrous oxide emissions because once formed, nitrous oxide needed little energy to transform into nitrogen.
Single atom alloy catalysts like this one are a subset of single atom catalysts, which usually involve single atoms supported on an inert support that doesn’t play a role in the catalytic transformation. That’s not the case here.
‘Delightedly, not only do the isolated active atoms in the single atom alloy catalyst demonstrate catalytic performance, but the surrounding metal atoms could affect the catalytic activity or selectivity – this alloying could lead to better performance than that from just single palladium atoms,’ comments Qing Jiang, an automobile materials expert based at Jilin University, China. Single atom alloys may also be easier to synthesise than other single atom catalysts. ‘The single metal atom sites you get are much better defined than single atoms dispersed in, for example, oxide supports, in which you may have strong metal–support interactions and form ionic species,’ says Stamatakis. He adds that this study is an important step toward commercial applications of single atom alloy catalysts. The high surface energy of single atoms means that single atom catalysts, in which the single atoms are isolated atoms anchored on the solid support, are less stable. Single atom alloy catalysts on the other hand should be more stable.
Most catalysts bind reactants and intermediates enough to break bonds within the molecule, but not so much that the product cannot escape. Single atom alloys, however, can sidestep this tradition: the reactants and products bind in different ways with the two atom types, for example allowing strong binding at the active site but weak binding elsewhere to allow the product to move away from the catalyst.
Jiang says the work could act as a foundation for designing single atom alloy catalysts in a different way. ‘It would be great to be able to predict bespoke bimetallic or multi-metallic combinations for given reactions of interest,’ adds Stamatakis. ‘This would save a lot of time in trial and error experimentation and would accelerate innovation in the field.’










No comments yet